当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, α-glycosidase inhibitory potential and molecular docking study of benzimidazole derivatives.
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2019-12-26 , DOI: 10.1016/j.bioorg.2019.103555 Muhammad Taha 1 , Fazal Rahim 2 , Khalid Zaman 2 , Manikandan Selvaraj 3 , Nizam Uddin 4 , Rai Khalid Farooq 5 , Muhammad Nawaz 6 , Muhammad Sajid 7 , Faisal Nawaz 8 , Mohamad Ibrahim 1 , Khalid Mohammed Khan 9
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2019-12-26 , DOI: 10.1016/j.bioorg.2019.103555 Muhammad Taha 1 , Fazal Rahim 2 , Khalid Zaman 2 , Manikandan Selvaraj 3 , Nizam Uddin 4 , Rai Khalid Farooq 5 , Muhammad Nawaz 6 , Muhammad Sajid 7 , Faisal Nawaz 8 , Mohamad Ibrahim 1 , Khalid Mohammed Khan 9
Affiliation
|
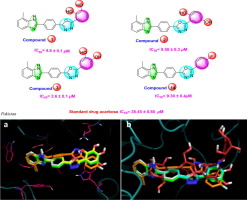
A series of twenty-six analogs of benzimidazole based oxadiazole have been synthesized and evaluated against alpha-glycosidase enzyme. Most the analogs showed excellent to good inhibitory potential. Among the screened analogs, analog 1, 2, 3 and 14 with IC50 values 4.6 ± 0.1, 9.50 ± 0.3, 2.6 ± 0.1 and 9.30 ± 0.4 µM respectively showedexcellent inhibitory potential than reference drug acarbose (IC50 = 38.45 ± 0.80 µM). Some of the analogs like 19, 21, 22 and 23 with methyl and methoxy substituent on phenyl ring show hydrophobic interaction and were found with no inhibitory potential. The binding interactions between synthesized analogs and ligands protein were confirmed through molecular docking study. Various spectroscopic techniques like 1H NMR, 13C NMR, and EI-MS were used for characterization of all synthesized analogs. These derivatives were synthesized by simple mode of synthesis like heterocyclic ring formation.
中文翻译:

苯并咪唑衍生物的合成,α-糖苷酶抑制潜力和分子对接研究。
已经合成了一系列二十六个基于苯并咪唑的恶二唑类似物,并针对α-糖苷酶进行了评估。大多数类似物显示出极好的抑制潜能。在筛选的类似物中,IC50值分别为4.6±0.1、9.50±0.3、2.6±0.1和9.30±0.4 µM的类似物1、2、3和14表现出比参比药物阿卡波糖优异的抑制潜力(IC50 = 38.45±0.80 µM)。在苯环上具有甲基和甲氧基取代基的某些类似物,例如19、21、22和23,表现出疏水性相互作用,并且没有抑制潜力。通过分子对接研究证实了合成的类似物与配体蛋白之间的结合相互作用。各种光谱技术(例如1H NMR,13C NMR和EI-MS)用于表征所有合成的类似物。
更新日期:2019-12-27
中文翻译:

苯并咪唑衍生物的合成,α-糖苷酶抑制潜力和分子对接研究。
已经合成了一系列二十六个基于苯并咪唑的恶二唑类似物,并针对α-糖苷酶进行了评估。大多数类似物显示出极好的抑制潜能。在筛选的类似物中,IC50值分别为4.6±0.1、9.50±0.3、2.6±0.1和9.30±0.4 µM的类似物1、2、3和14表现出比参比药物阿卡波糖优异的抑制潜力(IC50 = 38.45±0.80 µM)。在苯环上具有甲基和甲氧基取代基的某些类似物,例如19、21、22和23,表现出疏水性相互作用,并且没有抑制潜力。通过分子对接研究证实了合成的类似物与配体蛋白之间的结合相互作用。各种光谱技术(例如1H NMR,13C NMR和EI-MS)用于表征所有合成的类似物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号