当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Atomistic Understanding of Allosteric Inhibition of Glutamate Racemase: a Dampening of Native Activation Dynamics.
ChemMedChem ( IF 3.6 ) Pub Date : 2019-12-25 , DOI: 10.1002/cmdc.201900642 Katie R Witkin 1 , Nicholas R Vance 1 , Colleen Caldwell 2 , Quinn Li 2 , Liping Yu 2, 3 , M Ashley Spies 1, 2
ChemMedChem ( IF 3.6 ) Pub Date : 2019-12-25 , DOI: 10.1002/cmdc.201900642 Katie R Witkin 1 , Nicholas R Vance 1 , Colleen Caldwell 2 , Quinn Li 2 , Liping Yu 2, 3 , M Ashley Spies 1, 2
Affiliation
|
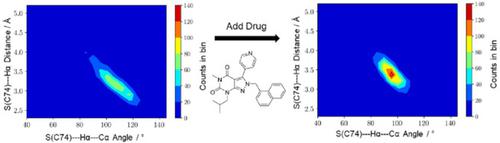
Glutamate racemases (GR) are members of the family of bacterial enzymes known as cofactor-independent racemases and epimerases and catalyze the stereoinversion of glutamate. D-amino acids are universally important for the proper construction of viable bacterial cell walls, and thus have been repeatedly validated as attractive targets for novel antimicrobial drug design. Significant aspects of the mechanism of this challenging stereoinversion remain unknown. The current study employs a combination of MD and QM/MM computational approaches to show that the GR from H. pylori must proceed via a pre-activation step, which is dependent on the enzyme's flexibility. This mechanism is starkly different from previously proposed mechanisms. These findings have immediate pharmaceutical relevance, as the H. pylori GR enzyme is a very attractive allosteric drug target. The results presented in this study offer a distinctly novel understanding of how AstraZeneca's lead series of inhibitors cripple the H. pylori GR's native motions, via prevention of this critical chemical pre-activation step. Our experimental studies, using SPR, fluorescence and NMR WaterLOGSY, show that H. pylori GR is not inhibited by the uncompetitive mechanism originally put forward by Lundqvist et al.. The current study supports a deep connection between native enzyme motions and chemical reactivity, which has strong relevance to the field of allosteric drug discovery.
中文翻译:

对谷氨酸消旋酶变构抑制的原子理解:天然激活动力学的抑制。
谷氨酸消旋酶 (GR) 是细菌酶家族的成员,被称为非辅因子依赖性消旋酶和差向异构酶,可催化谷氨酸的立体反转。 D-氨基酸对于活细菌细胞壁的正确构建普遍重要,因此已被反复验证为新型抗菌药物设计的有吸引力的靶标。这种具有挑战性的立体反转机制的重要方面仍然未知。目前的研究采用 MD 和 QM/MM 计算方法相结合,表明幽门螺杆菌的 GR 必须通过预激活步骤进行,该步骤取决于酶的灵活性。该机制与之前提出的机制截然不同。这些发现具有直接的药物相关性,因为幽门螺杆菌 GR 酶是一个非常有吸引力的变构药物靶点。这项研究中提出的结果为阿斯利康的领先系列抑制剂如何通过阻止这一关键的化学预激活步骤来削弱幽门螺杆菌 GR 的天然运动提供了全新的理解。我们使用 SPR、荧光和 NMR WaterLOGSY 的实验研究表明,幽门螺杆菌 GR 不受 Lundqvist 等人最初提出的非竞争性机制的抑制。当前的研究支持天然酶运动和化学反应性之间的深层联系,这与变构药物发现领域具有很强的相关性。
更新日期:2020-01-22
中文翻译:

对谷氨酸消旋酶变构抑制的原子理解:天然激活动力学的抑制。
谷氨酸消旋酶 (GR) 是细菌酶家族的成员,被称为非辅因子依赖性消旋酶和差向异构酶,可催化谷氨酸的立体反转。 D-氨基酸对于活细菌细胞壁的正确构建普遍重要,因此已被反复验证为新型抗菌药物设计的有吸引力的靶标。这种具有挑战性的立体反转机制的重要方面仍然未知。目前的研究采用 MD 和 QM/MM 计算方法相结合,表明幽门螺杆菌的 GR 必须通过预激活步骤进行,该步骤取决于酶的灵活性。该机制与之前提出的机制截然不同。这些发现具有直接的药物相关性,因为幽门螺杆菌 GR 酶是一个非常有吸引力的变构药物靶点。这项研究中提出的结果为阿斯利康的领先系列抑制剂如何通过阻止这一关键的化学预激活步骤来削弱幽门螺杆菌 GR 的天然运动提供了全新的理解。我们使用 SPR、荧光和 NMR WaterLOGSY 的实验研究表明,幽门螺杆菌 GR 不受 Lundqvist 等人最初提出的非竞争性机制的抑制。当前的研究支持天然酶运动和化学反应性之间的深层联系,这与变构药物发现领域具有很强的相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号