当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A light-triggered self-reinforced nanoagent for targeted chemo-photodynamic therapy of breast cancer bone metastases via ER stress and mitochondria mediated apoptotic pathways.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-12-25 , DOI: 10.1016/j.jconrel.2019.12.043 Yanjuan Huang 1 , Zhanghong Xiao 1 , Zilin Guan 1 , Yifeng Shen 1 , Yali Jiang 1 , Xiaoyu Xu 1 , Zeqian Huang 1 , Chunshun Zhao 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-12-25 , DOI: 10.1016/j.jconrel.2019.12.043 Yanjuan Huang 1 , Zhanghong Xiao 1 , Zilin Guan 1 , Yifeng Shen 1 , Yali Jiang 1 , Xiaoyu Xu 1 , Zeqian Huang 1 , Chunshun Zhao 1
Affiliation
|
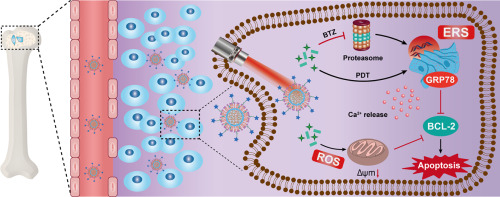
Current therapeutic strategies for the treatment of bone metastases are often limited by the lack of selectivity, severe systemic toxicity and suboptimal efficacy. Nanomedicine meditated chemo-photodynamic therapy provides a promising therapeutic opportunity for enhanced cancer therapy. Herein, we constructed an alendronate (ALN)-functionalized bone-seeking nanoagent (BTZ@ZnPc-ALN) to co-deliver the proteasome inhibitor bortezomib (BTZ) and the photosensitizer Zinc phthalocyanine (ZnPc) for synergistic chemo-photodynamic therapy of bone metastases. Results showed that BTZ@ZnPc-ALN possessed favorable bone affinity both in vitro and in vivo and could release drug in a pH-responsive manner. Under irradiation, BTZ@ZnPc-ALN could generate reactive oxygen species (ROS) to cause mitochondrial damage, and increase the cytosolic Ca2+ levels and the expression of GRP78 protein to induce excessive endoplasmic reticulum (ER) stress, thereby synergistically inhibiting cell proliferation. More importantly, BTZ@ZnPc-ALN could prolong blood circulation time and preferentially navigate to the bone affected site. As a result, tumor growth was significantly inhibited by bone targeted chemo-photodynamic therapy, with tumor volume cut down by 85% compared with PBS group and bone remained undamaged. Besides, the systemic toxicity of BTZ was significantly reduced. Therefore, the versatile nanoagent is expected to be a promising nanoplatform to concern multiple intracellular stress for remarkable synergistic chemo-photodynamic therapy of bone metastases.
中文翻译:

一种光触发的自增强纳米剂,用于通过内质网应激和线粒体介导的凋亡途径对乳腺癌骨转移进行化学光动力学靶向治疗。
缺乏选择性,严重的全身毒性和次优的疗效常常限制了目前治疗骨转移的治疗策略。纳米医学思考的化学光动力疗法为增强癌症治疗提供了有希望的治疗机会。本文中,我们构建了一种阿仑膦酸盐(ALN)功能化的寻骨纳米剂(BTZ @ ZnPc-ALN),以共同提供蛋白酶体抑制剂硼替佐米(BTZ)和光敏剂酞菁锌(ZnPc),用于骨转移的协同化学光动力学治疗。 。结果表明,BTZ @ ZnPc-ALN在体内和体外均具有良好的骨亲和力,并能以pH响应的方式释放药物。在辐照下,BTZ @ ZnPc-ALN可能产生活性氧(ROS)从而引起线粒体损伤,并增加胞质Ca2 +水平和GRP78蛋白的表达,以诱导过度的内质网(ER)应激,从而协同抑制细胞增殖。更重要的是,BTZ @ ZnPc-ALN可以延长血液循环时间,并优先导航到患骨部位。结果,骨靶向化学光动力疗法显着抑制了肿瘤的生长,与PBS组相比,肿瘤体积减少了85%,并且骨骼保持未受损。此外,BTZ的全身毒性显着降低。因此,多功能纳米剂有望成为一种有前途的纳米平台,涉及多种细胞内应力,用于骨转移的显着协同化学光动力疗法。从而协同抑制细胞增殖。更重要的是,BTZ @ ZnPc-ALN可以延长血液循环时间,并优先导航到患骨部位。结果,骨靶向化学光动力疗法显着抑制了肿瘤的生长,与PBS组相比,肿瘤体积减少了85%,并且骨骼保持未受损。此外,BTZ的全身毒性显着降低。因此,多功能纳米剂有望成为一种有前途的纳米平台,涉及多种细胞内应力,用于骨转移的显着协同化学光动力疗法。从而协同抑制细胞增殖。更重要的是,BTZ @ ZnPc-ALN可以延长血液循环时间,并优先导航到患骨部位。结果,骨靶向化学光动力疗法显着抑制了肿瘤的生长,与PBS组相比,肿瘤体积减少了85%,并且骨骼保持未受损。此外,BTZ的全身毒性显着降低。因此,多功能纳米剂有望成为一种有前途的纳米平台,涉及多种细胞内应力,用于骨转移的显着协同化学光动力疗法。骨靶向化学光动力疗法显着抑制了肿瘤的生长,与PBS组相比,肿瘤体积减少了85%,并且骨骼保持未受损。此外,BTZ的全身毒性显着降低。因此,多功能纳米剂有望成为一种有前途的纳米平台,涉及多种细胞内应力,用于骨转移的显着协同化学光动力疗法。骨靶向化学光动力疗法显着抑制了肿瘤的生长,与PBS组相比,肿瘤体积减少了85%,并且骨骼保持未受损。此外,BTZ的全身毒性显着降低。因此,多功能纳米剂有望成为一种有前途的纳米平台,涉及多种细胞内应力,用于骨转移的显着协同化学光动力疗法。
更新日期:2019-12-26
中文翻译:

一种光触发的自增强纳米剂,用于通过内质网应激和线粒体介导的凋亡途径对乳腺癌骨转移进行化学光动力学靶向治疗。
缺乏选择性,严重的全身毒性和次优的疗效常常限制了目前治疗骨转移的治疗策略。纳米医学思考的化学光动力疗法为增强癌症治疗提供了有希望的治疗机会。本文中,我们构建了一种阿仑膦酸盐(ALN)功能化的寻骨纳米剂(BTZ @ ZnPc-ALN),以共同提供蛋白酶体抑制剂硼替佐米(BTZ)和光敏剂酞菁锌(ZnPc),用于骨转移的协同化学光动力学治疗。 。结果表明,BTZ @ ZnPc-ALN在体内和体外均具有良好的骨亲和力,并能以pH响应的方式释放药物。在辐照下,BTZ @ ZnPc-ALN可能产生活性氧(ROS)从而引起线粒体损伤,并增加胞质Ca2 +水平和GRP78蛋白的表达,以诱导过度的内质网(ER)应激,从而协同抑制细胞增殖。更重要的是,BTZ @ ZnPc-ALN可以延长血液循环时间,并优先导航到患骨部位。结果,骨靶向化学光动力疗法显着抑制了肿瘤的生长,与PBS组相比,肿瘤体积减少了85%,并且骨骼保持未受损。此外,BTZ的全身毒性显着降低。因此,多功能纳米剂有望成为一种有前途的纳米平台,涉及多种细胞内应力,用于骨转移的显着协同化学光动力疗法。从而协同抑制细胞增殖。更重要的是,BTZ @ ZnPc-ALN可以延长血液循环时间,并优先导航到患骨部位。结果,骨靶向化学光动力疗法显着抑制了肿瘤的生长,与PBS组相比,肿瘤体积减少了85%,并且骨骼保持未受损。此外,BTZ的全身毒性显着降低。因此,多功能纳米剂有望成为一种有前途的纳米平台,涉及多种细胞内应力,用于骨转移的显着协同化学光动力疗法。从而协同抑制细胞增殖。更重要的是,BTZ @ ZnPc-ALN可以延长血液循环时间,并优先导航到患骨部位。结果,骨靶向化学光动力疗法显着抑制了肿瘤的生长,与PBS组相比,肿瘤体积减少了85%,并且骨骼保持未受损。此外,BTZ的全身毒性显着降低。因此,多功能纳米剂有望成为一种有前途的纳米平台,涉及多种细胞内应力,用于骨转移的显着协同化学光动力疗法。骨靶向化学光动力疗法显着抑制了肿瘤的生长,与PBS组相比,肿瘤体积减少了85%,并且骨骼保持未受损。此外,BTZ的全身毒性显着降低。因此,多功能纳米剂有望成为一种有前途的纳米平台,涉及多种细胞内应力,用于骨转移的显着协同化学光动力疗法。骨靶向化学光动力疗法显着抑制了肿瘤的生长,与PBS组相比,肿瘤体积减少了85%,并且骨骼保持未受损。此外,BTZ的全身毒性显着降低。因此,多功能纳米剂有望成为一种有前途的纳米平台,涉及多种细胞内应力,用于骨转移的显着协同化学光动力疗法。









































 京公网安备 11010802027423号
京公网安备 11010802027423号