Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2019-12-18 , DOI: 10.1016/j.jcou.2019.11.026 Márton Kőrösi , János Béri , Alina Hanu , Sabine Kareth , Edit Székely
|
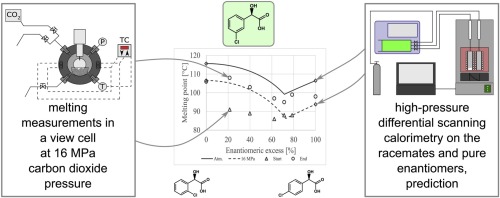
Chiral molecules pose great research potential related to their wide applications in various industries due to the different biological effects of enantiomers. Chiral temperature-composition (melting) phase diagrams are widely available for atmospheric pressure, and those are frequently applied to predict the maximal efficiency of enantioseparation. However, self-disproportionation of enantiomers has already been observed in gas antisolvent fractionation as well. Here, we present the first chiral temperature-composition phase diagrams under carbon dioxide pressure. Chiral temperature-composition phase diagrams of 2-, 3- and 4-chloromandelic acids were determined experimentally. High pressure differential scanning calorimetry and the visual first and last melting point method were used simultaneously to determine the temperature-composition phase diagrams of the compounds. Results were compared to the atmospheric phase diagrams. At 16 and 20 MPa pressures modest melting point depressions of 10−18 °C were observed for any enantiomeric compositions, while the eutectic composition remained practically unaffected. The Schröder–van Laar and Prigogine–Defay equations, widely accepted for the prediction of atmospheric temperature-composition phase diagrams of enantiomeric mixtures, were proved to be applicable also in the presence of a soluble pressurized gas.
中文翻译:

手性化合物的高压熔融平衡:二氧化碳气氛下氯化扁桃酸的实践研究
由于对映异构体的不同生物作用,手性分子由于其在各种工业中的广泛应用而具有巨大的研究潜力。手性温度组成(熔融)相图可广泛用于大气压,并且经常用于预测对映体分离的最大效率。然而,在气体反溶剂分馏中也已经观察到对映体的自歧化。在这里,我们介绍了二氧化碳压力下的第一个手性温度组成相图。实验确定了2-,3-和4-氯扁桃酸的手性温度组成相图。同时使用高压差示扫描量热法和可见的第一个和最后一个熔点方法来确定化合物的温度组成相图。将结果与大气相图进行了比较。在任何压力下,在16和20 MPa的压力下,任何对映体组成都观察到10-18°C的适度熔点降低,而共晶组成实际上不受影响。事实证明,Schröder-vanLaar和Prigogine-Defay方程广泛用于预测对映体混合物的大气温度-成分相图,也适用于存在加压气体的情况。在任何压力下,在16和20 MPa的压力下,任何对映体组成都观察到10-18°C的适度熔点降低,而共晶组成实际上不受影响。事实证明,Schröder-vanLaar和Prigogine-Defay方程广泛用于预测对映体混合物的大气温度-成分相图,也适用于存在加压气体的情况。在任何压力下,在16和20 MPa的压力下,任何对映体组成都观察到10-18°C的适度熔点降低,而共晶组成实际上不受影响。事实证明,Schröder-vanLaar和Prigogine-Defay方程广泛用于预测对映体混合物的大气温度-成分相图,也适用于存在加压气体的情况。











































 京公网安备 11010802027423号
京公网安备 11010802027423号