Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
H2A.Z facilitates licensing and activation of early replication origins
Nature ( IF 50.5 ) Pub Date : 2019-12-25 , DOI: 10.1038/s41586-019-1877-9 Haizhen Long 1, 2 , Liwei Zhang 1 , Mengjie Lv 3 , Zengqi Wen 1, 2 , Wenhao Zhang 4 , Xiulan Chen 2, 5 , Peitao Zhang 6 , Tongqing Li 7 , Luyuan Chang 1, 2 , Caiwei Jin 2, 3 , Guozhao Wu 1, 2 , Xi Wang 8 , Fuquan Yang 2, 5 , Jianfeng Pei 7 , Ping Chen 1 , Raphael Margueron 9 , Haiteng Deng 4 , Mingzhao Zhu 2, 3 , Guohong Li 1, 2
Nature ( IF 50.5 ) Pub Date : 2019-12-25 , DOI: 10.1038/s41586-019-1877-9 Haizhen Long 1, 2 , Liwei Zhang 1 , Mengjie Lv 3 , Zengqi Wen 1, 2 , Wenhao Zhang 4 , Xiulan Chen 2, 5 , Peitao Zhang 6 , Tongqing Li 7 , Luyuan Chang 1, 2 , Caiwei Jin 2, 3 , Guozhao Wu 1, 2 , Xi Wang 8 , Fuquan Yang 2, 5 , Jianfeng Pei 7 , Ping Chen 1 , Raphael Margueron 9 , Haiteng Deng 4 , Mingzhao Zhu 2, 3 , Guohong Li 1, 2
Affiliation
|
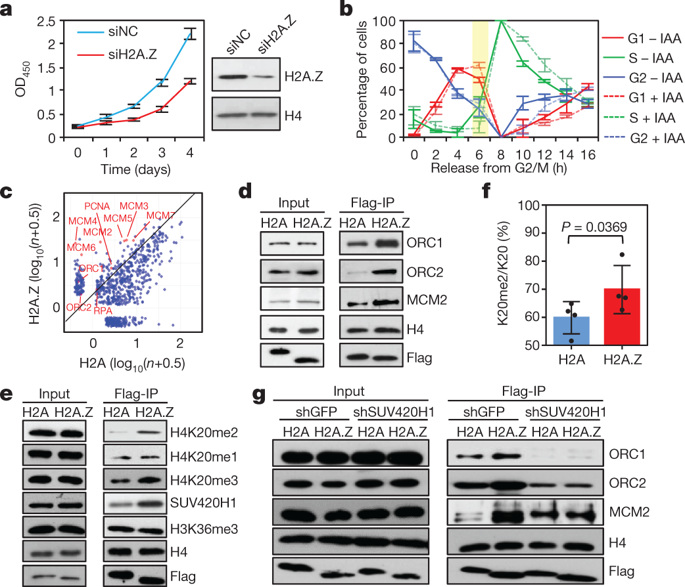
DNA replication is a tightly regulated process that ensures the precise duplication of the genome during the cell cycle 1 . In eukaryotes, the licensing and activation of replication origins are regulated by both DNA sequence and chromatin features 2 . However, the chromatin-based regulatory mechanisms remain largely uncharacterized. Here we show that, in HeLa cells, nucleosomes containing the histone variant H2A.Z are enriched with histone H4 that is dimethylated on its lysine 20 residue (H4K20me2) and with bound origin-recognition complex (ORC). In vitro studies show that H2A.Z-containing nucleosomes bind directly to the histone lysine methyltransferase enzyme SUV420H1, promoting H4K20me2 deposition, which is in turn required for ORC1 binding. Genome-wide studies show that signals from H4K20me2, ORC1 and nascent DNA strands co-localize with H2A.Z, and that depletion of H2A.Z results in decreased H4K20me2, ORC1 and nascent-strand signals throughout the genome. H2A.Z-regulated replication origins have a higher firing efficiency and early replication timing compared with other origins. Our results suggest that the histone variant H2A.Z epigenetically regulates the licensing and activation of early replication origins and maintains replication timing through the SUV420H1–H4K20me2–ORC1 axis. DNA replication in eukaryotes requires the histone variant H2A.Z, which binds the enzyme SUV420H1 to promote the dimethylation of histone H4, in turn recruiting the origin-recognition complex to activate early replication origins.
中文翻译:

H2A.Z 促进了早期复制源的许可和激活
DNA 复制是一个严格调控的过程,可确保在细胞周期 1 中精确复制基因组。在真核生物中,复制起点的许可和激活受 DNA 序列和染色质特征 2 的调节。然而,基于染色质的调控机制在很大程度上仍未得到表征。在这里,我们表明,在 HeLa 细胞中,含有组蛋白变体 H2A.Z 的核小体富含组蛋白 H4,组蛋白 H4 在其赖氨酸 20 残基 (H4K20me2) 上二甲基化,并具有结合的起源识别复合物 (ORC)。体外研究表明,含有 H2A.Z 的核小体直接与组蛋白赖氨酸甲基转移酶 SUV420H1 结合,促进 H4K20me2 沉积,而这又是 ORC1 结合所必需的。全基因组研究表明,来自 H4K20me2、ORC1 和新生 DNA 链的信号与 H2A 共定位。Z,而 H2A.Z 的消耗导致整个基因组中的 H4K20me2、ORC1 和新生链信号减少。与其他来源相比,H2A.Z 调节的复制起点具有更高的激发效率和早期复制时间。我们的研究结果表明,组蛋白变体 H2A.Z 表观遗传调节早期复制起点的许可和激活,并通过 SUV420H1-H4K20me2-ORC1 轴维持复制时间。真核生物中的 DNA 复制需要组蛋白变体 H2A.Z,它与酶 SUV420H1 结合以促进组蛋白 H4 的二甲基化,进而募集起源识别复合物以激活早期复制起点。与其他来源相比,Z 调节的复制起点具有更高的激发效率和早期复制时间。我们的研究结果表明,组蛋白变体 H2A.Z 表观遗传调节早期复制起点的许可和激活,并通过 SUV420H1-H4K20me2-ORC1 轴维持复制时间。真核生物中的 DNA 复制需要组蛋白变体 H2A.Z,它与酶 SUV420H1 结合以促进组蛋白 H4 的二甲基化,进而募集起源识别复合物以激活早期复制起点。与其他来源相比,Z 调节的复制起点具有更高的激发效率和早期复制时间。我们的研究结果表明,组蛋白变体 H2A.Z 表观遗传调节早期复制起点的许可和激活,并通过 SUV420H1-H4K20me2-ORC1 轴维持复制时间。真核生物中的 DNA 复制需要组蛋白变体 H2A.Z,它与酶 SUV420H1 结合以促进组蛋白 H4 的二甲基化,进而募集起源识别复合物以激活早期复制起点。
更新日期:2019-12-25
中文翻译:

H2A.Z 促进了早期复制源的许可和激活
DNA 复制是一个严格调控的过程,可确保在细胞周期 1 中精确复制基因组。在真核生物中,复制起点的许可和激活受 DNA 序列和染色质特征 2 的调节。然而,基于染色质的调控机制在很大程度上仍未得到表征。在这里,我们表明,在 HeLa 细胞中,含有组蛋白变体 H2A.Z 的核小体富含组蛋白 H4,组蛋白 H4 在其赖氨酸 20 残基 (H4K20me2) 上二甲基化,并具有结合的起源识别复合物 (ORC)。体外研究表明,含有 H2A.Z 的核小体直接与组蛋白赖氨酸甲基转移酶 SUV420H1 结合,促进 H4K20me2 沉积,而这又是 ORC1 结合所必需的。全基因组研究表明,来自 H4K20me2、ORC1 和新生 DNA 链的信号与 H2A 共定位。Z,而 H2A.Z 的消耗导致整个基因组中的 H4K20me2、ORC1 和新生链信号减少。与其他来源相比,H2A.Z 调节的复制起点具有更高的激发效率和早期复制时间。我们的研究结果表明,组蛋白变体 H2A.Z 表观遗传调节早期复制起点的许可和激活,并通过 SUV420H1-H4K20me2-ORC1 轴维持复制时间。真核生物中的 DNA 复制需要组蛋白变体 H2A.Z,它与酶 SUV420H1 结合以促进组蛋白 H4 的二甲基化,进而募集起源识别复合物以激活早期复制起点。与其他来源相比,Z 调节的复制起点具有更高的激发效率和早期复制时间。我们的研究结果表明,组蛋白变体 H2A.Z 表观遗传调节早期复制起点的许可和激活,并通过 SUV420H1-H4K20me2-ORC1 轴维持复制时间。真核生物中的 DNA 复制需要组蛋白变体 H2A.Z,它与酶 SUV420H1 结合以促进组蛋白 H4 的二甲基化,进而募集起源识别复合物以激活早期复制起点。与其他来源相比,Z 调节的复制起点具有更高的激发效率和早期复制时间。我们的研究结果表明,组蛋白变体 H2A.Z 表观遗传调节早期复制起点的许可和激活,并通过 SUV420H1-H4K20me2-ORC1 轴维持复制时间。真核生物中的 DNA 复制需要组蛋白变体 H2A.Z,它与酶 SUV420H1 结合以促进组蛋白 H4 的二甲基化,进而募集起源识别复合物以激活早期复制起点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号