当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microwave‐assisted rapid and efficient synthesis of chromene‐fused pyrrole derivatives through multicomponent reaction and evaluation of antibacterial activity with molecular docking investigation
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2019-12-25 , DOI: 10.1002/jhet.3773 Nilofar Baral 1 , Deepak Ranjan Mishra 1 , Nilima Priyadarsini Mishra 1 , Seetaram Mohapatra 1 , Bishnu Prasad Raiguru 1 , Pravati Panda 1 , Sabita Nayak 1 , Mukesh Nayak 1 , P. Sudhir Kumar 2
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2019-12-25 , DOI: 10.1002/jhet.3773 Nilofar Baral 1 , Deepak Ranjan Mishra 1 , Nilima Priyadarsini Mishra 1 , Seetaram Mohapatra 1 , Bishnu Prasad Raiguru 1 , Pravati Panda 1 , Sabita Nayak 1 , Mukesh Nayak 1 , P. Sudhir Kumar 2
Affiliation
|
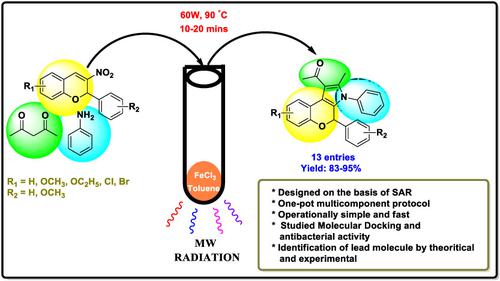
The current study aimed to identify a new strategy of FeCl3 catalyzed multicomponent synthesis of substituted 2H‐chromene–fused pyrrole derivatives. A series of chromene‐based pyrrole prepared by employing an array of 3‐nitro‐2H‐chromenes, aniline, and acetylacetone in toluene under microwave irradiation. Using FeCl3 as a prompt catalyst and microwave irradiation to synthesize 2H‐chromene–fused pyrrole motifs significantly reduces the reaction time and facilitates to high yields (83%‐95%). Structure of all synthesized compounds analyzed by spectroscopic analysis. One‐pot reaction, short reaction period, and simple experimental procedure are the fascinating properties associated with this protocol. The in vitro antibacterial activity of the entire series was assessed against Staphylococcus aureus and Escherichia coli. Out of all the compounds, 15b and 15h revealed most excellent potency against both the bacterial strains relative to the reference gentamicin. Docking study was employed to determine the possible binding orientation of DNA gyrase with the active sites of chromene‐fused pyrrole analog. The docking results show that compounds 15b and 15h have higher binding affinity with energy −8.00 and −8.80 kcal/mol. These results illuminate the mode of binding progression and provide an esteemed pathway for the design and the structural modification of chromene‐fused pyrroles as a newly advanced class of antibacterial agent.
中文翻译:

微波辅助的多组分反应快速高效合成苯并二甲基吡咯衍生物,并通过分子对接研究评估其抗菌活性
当前的研究旨在确定FeCl 3催化取代2 H-色烯稠合的吡咯衍生物多组分合成的新策略。在微波辐射下,通过在甲苯中使用一系列3-硝基-2 H-苯甲基,苯胺和乙酰丙酮制备了一系列基于苯甲基的吡咯。使用FeCl 3作为快速催化剂,并通过微波辐射合成2 H色烯-融合的吡咯基序可显着缩短反应时间,并有助于提高收率(83%-95%)。通过光谱分析分析所有合成化合物的结构。单罐反应,较短的反应时间和简单的实验程序是与此协议相关的令人着迷的特性。评估了整个系列对金黄色葡萄球菌和大肠杆菌的体外抗菌活性。在所有化合物中,15b和15h相对于参考庆大霉素,它显示出对两种细菌菌株的最优异的效力。通过对接研究确定了DNA促旋酶与色烯融合的吡咯类似物的活性位点的可能结合方向。对接结果表明,化合物15b和15h具有更高的结合亲和力,能量为-8.00和-8.80 kcal / mol。这些结果阐明了结合过程的模式,并为设计和新型的苯并二氢吡咯作为一种新型抗菌剂的结构修饰提供了一条备受推崇的途径。
更新日期:2019-12-26
中文翻译:

微波辅助的多组分反应快速高效合成苯并二甲基吡咯衍生物,并通过分子对接研究评估其抗菌活性
当前的研究旨在确定FeCl 3催化取代2 H-色烯稠合的吡咯衍生物多组分合成的新策略。在微波辐射下,通过在甲苯中使用一系列3-硝基-2 H-苯甲基,苯胺和乙酰丙酮制备了一系列基于苯甲基的吡咯。使用FeCl 3作为快速催化剂,并通过微波辐射合成2 H色烯-融合的吡咯基序可显着缩短反应时间,并有助于提高收率(83%-95%)。通过光谱分析分析所有合成化合物的结构。单罐反应,较短的反应时间和简单的实验程序是与此协议相关的令人着迷的特性。评估了整个系列对金黄色葡萄球菌和大肠杆菌的体外抗菌活性。在所有化合物中,15b和15h相对于参考庆大霉素,它显示出对两种细菌菌株的最优异的效力。通过对接研究确定了DNA促旋酶与色烯融合的吡咯类似物的活性位点的可能结合方向。对接结果表明,化合物15b和15h具有更高的结合亲和力,能量为-8.00和-8.80 kcal / mol。这些结果阐明了结合过程的模式,并为设计和新型的苯并二氢吡咯作为一种新型抗菌剂的结构修饰提供了一条备受推崇的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号