当前位置:
X-MOL 学术
›
Mol. Ther. Methods Clin. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Targeting Antigen to the Surface of EVs Improves the In Vivo Immunogenicity of Human and Non-human Adenoviral Vaccines in Mice.
Molecular Therapy - Methods & Clinical Development ( IF 4.7 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.omtm.2019.12.003 Carly M Bliss 1 , Andrea J Parsons 1 , Raffael Nachbagauer 1 , Jennifer R Hamilton 1, 2 , Federica Cappuccini 3 , Marta Ulaszewska 3 , Jason P Webber 4 , Aled Clayton 4 , Adrian V S Hill 3 , Lynda Coughlan 1, 3
Molecular Therapy - Methods & Clinical Development ( IF 4.7 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.omtm.2019.12.003 Carly M Bliss 1 , Andrea J Parsons 1 , Raffael Nachbagauer 1 , Jennifer R Hamilton 1, 2 , Federica Cappuccini 3 , Marta Ulaszewska 3 , Jason P Webber 4 , Aled Clayton 4 , Adrian V S Hill 3 , Lynda Coughlan 1, 3
Affiliation
|
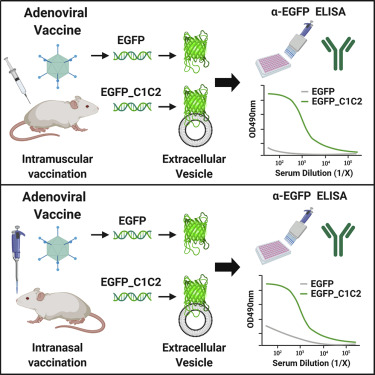
Adenoviral (Ad) vectors represent promising vaccine platforms for infectious disease. To overcome pre-existing immunity to commonly used human adenovirus serotype 5 (Ad5), vectors based on rare species or non-human Ads are being developed. However, these vectors often exhibit reduced potency compared with Ad5, necessitating the use of innovative approaches to augment the immunogenicity of the encoded antigen (Ag). To achieve this, we engineered model Ag, enhanced green fluorescent protein (EGFP), for targeting to the surface of host-derived extracellular vesicles (EVs), namely exosomes. Exosomes are nano-sized EVs that play important roles in cell-to-cell communication and in regulating immune responses. Directed targeting of Ag to the surface of EVs/exosomes is achieved by "exosome display," through fusion of Ag to the C1C2 domain of lactadherin, a protein highly enriched in exosomes. Herein, we engineered chimpanzee adenovirus ChAdOx1 and Ad5-based vaccines encoding EGFP, or EGFP targeted to EVs (EGFP_C1C2), and compared vaccine immunogenicity in mice. We determined that exosome display substantially increases Ag-specific humoral immunity following intramuscular and intranasal vaccination, improving the immunological potency of both ChAdOx1 and Ad5. We propose that this Ag-engineering approach could increase the immunogenicity of diverse Ad vectors that exhibit desirable manufacturing characteristics, but currently lack the potency of Ad5.
中文翻译:

将抗原靶向电动汽车表面可改善小鼠中人类和非人类腺病毒疫苗的体内免疫原性。
腺病毒(Ad)载体代表了有希望的传染病疫苗平台。为了克服对常用的人类腺病毒血清型5(Ad5)的现有免疫力,正在开发基于稀有物种或非人类Ads的载体。但是,与Ad5相比,这些载体的效能通常降低,因此有必要使用创新的方法来增强编码抗原(Ag)的免疫原性。为了实现这一目标,我们设计了模型Ag,增强型绿色荧光蛋白(EGFP),用于靶向宿主来源的细胞外囊泡(EVs)(即外泌体)的表面。外泌体是纳米级的电动汽车,在细胞与细胞之间的交流和调节免疫反应中起重要作用。通过“外泌体展示”可将Ag直接靶向到EV /外泌体表面,通过将Ag融合到乳腺粘附素的C1C2结构域上,而乳粘附素是高度富含外泌体的蛋白质。本文中,我们设计了编码EGFP或靶向EV的EGFP(EGFP_C1C2)的黑猩猩腺病毒ChAdOx1和基于Ad5的疫苗,并比较了小鼠的疫苗免疫原性。我们确定,外泌体展示可在肌肉内和鼻内疫苗接种后显着增加Ag特异性体液免疫,从而改善ChAdOx1和Ad5的免疫效力。我们建议这种Ag工程方法可以提高具有理想制造特性但目前缺乏Ad5效力的各种Ad载体的免疫原性。并比较了小鼠的疫苗免疫原性。我们确定,外泌体展示可在肌肉内和鼻内疫苗接种后显着增加Ag特异性体液免疫,从而改善ChAdOx1和Ad5的免疫效力。我们建议这种Ag工程方法可以提高具有理想制造特性但目前缺乏Ad5效力的各种Ad载体的免疫原性。并比较了小鼠的疫苗免疫原性。我们确定,外泌体展示可在肌肉内和鼻内疫苗接种后显着增加Ag特异性体液免疫,从而改善ChAdOx1和Ad5的免疫效力。我们建议这种Ag工程方法可以提高具有理想制造特性但目前缺乏Ad5效力的各种Ad载体的免疫原性。
更新日期:2019-12-25
中文翻译:

将抗原靶向电动汽车表面可改善小鼠中人类和非人类腺病毒疫苗的体内免疫原性。
腺病毒(Ad)载体代表了有希望的传染病疫苗平台。为了克服对常用的人类腺病毒血清型5(Ad5)的现有免疫力,正在开发基于稀有物种或非人类Ads的载体。但是,与Ad5相比,这些载体的效能通常降低,因此有必要使用创新的方法来增强编码抗原(Ag)的免疫原性。为了实现这一目标,我们设计了模型Ag,增强型绿色荧光蛋白(EGFP),用于靶向宿主来源的细胞外囊泡(EVs)(即外泌体)的表面。外泌体是纳米级的电动汽车,在细胞与细胞之间的交流和调节免疫反应中起重要作用。通过“外泌体展示”可将Ag直接靶向到EV /外泌体表面,通过将Ag融合到乳腺粘附素的C1C2结构域上,而乳粘附素是高度富含外泌体的蛋白质。本文中,我们设计了编码EGFP或靶向EV的EGFP(EGFP_C1C2)的黑猩猩腺病毒ChAdOx1和基于Ad5的疫苗,并比较了小鼠的疫苗免疫原性。我们确定,外泌体展示可在肌肉内和鼻内疫苗接种后显着增加Ag特异性体液免疫,从而改善ChAdOx1和Ad5的免疫效力。我们建议这种Ag工程方法可以提高具有理想制造特性但目前缺乏Ad5效力的各种Ad载体的免疫原性。并比较了小鼠的疫苗免疫原性。我们确定,外泌体展示可在肌肉内和鼻内疫苗接种后显着增加Ag特异性体液免疫,从而改善ChAdOx1和Ad5的免疫效力。我们建议这种Ag工程方法可以提高具有理想制造特性但目前缺乏Ad5效力的各种Ad载体的免疫原性。并比较了小鼠的疫苗免疫原性。我们确定,外泌体展示可在肌肉内和鼻内疫苗接种后显着增加Ag特异性体液免疫,从而改善ChAdOx1和Ad5的免疫效力。我们建议这种Ag工程方法可以提高具有理想制造特性但目前缺乏Ad5效力的各种Ad载体的免疫原性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号