当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
RAB18 Loss Interferes With Lipid Droplet Catabolism and Provokes Autophagy Network Adaptations.
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.jmb.2019.12.031 Fazilet Bekbulat 1 , Daniel Schmitt 1 , Anne Feldmann 1 , Heike Huesmann 1 , Stefan Eimer 2 , Thomas Juretschke 3 , Petra Beli 3 , Christian Behl 1 , Andreas Kern 1
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.jmb.2019.12.031 Fazilet Bekbulat 1 , Daniel Schmitt 1 , Anne Feldmann 1 , Heike Huesmann 1 , Stefan Eimer 2 , Thomas Juretschke 3 , Petra Beli 3 , Christian Behl 1 , Andreas Kern 1
Affiliation
|
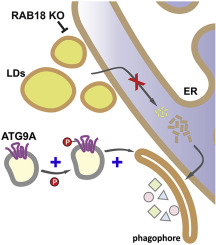
Autophagy is dependent on appropriate lipid supply for autophagosome formation. The regulation of lipid acquisition and the autophagy network response to lipid-limiting conditions are mostly elusive. Here, we show that the knockout of the RAB GTPase RAB18 interferes with lipid droplet catabolism, causing an impaired fatty acid release. The resulting reduced lipid-droplet-derived lipid availability influences autophagy and provokes adaptive modifications of the autophagy network. These adjustments include increased expression and phosphorylation of ATG2B as well as augmented formation of the ATG12-ATG5 conjugate. Moreover, ATG9A shows an enhanced phosphorylation at amino acid residues tyrosine 8 and serine 14, resulting in an increased ATG9A trafficking. Via pharmacological inhibition of Y8 phosphorylation, we demonstrate that this ATG9A modification is important to maintain basal autophagy under RAB18 knockout conditions. However, while the network adaptations are sufficient to maintain basal autophagic activity, they are incapable of ensuring autophagy induction upon starvation, which is characterized by an enhanced lipid demand. Thus, here, we define the molecular role of RAB18 in connecting lipid droplets and autophagy, emphasize the significance of lipid droplets as lipid sources for the degradative pathway, and uncover a remarkable autophagy network plasticity, including phosphorylation-dependent ATG9A activation, to compensate reduced lipid availability in order to rescue basal autophagic activity.
中文翻译:

RAB18损失会干扰脂滴的代谢并引起自噬网络适应。
自噬依赖于适当的脂质供应来形成自噬体。脂质获取的调节和对脂质限制条件的自噬网络反应大多难以捉摸。在这里,我们显示了敲除RAB GTPase RAB18会干扰脂质滴的分解代谢,从而导致脂肪酸释放受损。由此产生的降低的脂质液滴来源的脂质利用率会影响自噬,并引起自噬网络的适应性修饰。这些调节包括ATG2B的表达和磷酸化增加以及ATG12-ATG5缀合物的形成增加。此外,ATG9A在氨基酸残基酪氨酸8和丝氨酸14处显示出增强的磷酸化作用,导致ATG9A转运增加。通过药理作用抑制Y8磷酸化,我们证明了这种ATG9A修饰对于在RAB18基因敲除条件下维持基础自噬很重要。然而,尽管网络适应足以维持基础自噬活性,但它们不能确保饥饿时自噬的诱导,其特征是脂质需求增加。因此,在这里,我们定义了RAB18在连接脂滴和自噬中的分子作用,强调了脂滴作为降解途径的脂源的重要性,并揭示了显着的自噬网络可塑性,包括磷酸化依赖性ATG9A活化,以补偿降低的脂质供应以拯救基础自噬活性。尽管网络适应足以维持基础自噬活性,但它们不能确保饥饿时自噬的诱导,其特征是脂质需求增加。因此,在这里,我们定义了RAB18在连接脂滴和自噬中的分子作用,强调了脂滴作为降解途径的脂源的重要性,并揭示了显着的自噬网络可塑性,包括磷酸化依赖性ATG9A活化,以补偿降低的脂质供应以拯救基础自噬活性。尽管网络适应足以维持基础自噬活性,但它们不能确保饥饿时自噬的诱导,其特征是脂质需求增加。因此,在这里,我们定义了RAB18在连接脂滴和自噬中的分子作用,强调了脂滴作为降解途径的脂源的重要性,并揭示了显着的自噬网络可塑性,包括磷酸化依赖性ATG9A活化,以补偿降低的脂质供应以拯救基础自噬活性。
更新日期:2019-12-25
中文翻译:

RAB18损失会干扰脂滴的代谢并引起自噬网络适应。
自噬依赖于适当的脂质供应来形成自噬体。脂质获取的调节和对脂质限制条件的自噬网络反应大多难以捉摸。在这里,我们显示了敲除RAB GTPase RAB18会干扰脂质滴的分解代谢,从而导致脂肪酸释放受损。由此产生的降低的脂质液滴来源的脂质利用率会影响自噬,并引起自噬网络的适应性修饰。这些调节包括ATG2B的表达和磷酸化增加以及ATG12-ATG5缀合物的形成增加。此外,ATG9A在氨基酸残基酪氨酸8和丝氨酸14处显示出增强的磷酸化作用,导致ATG9A转运增加。通过药理作用抑制Y8磷酸化,我们证明了这种ATG9A修饰对于在RAB18基因敲除条件下维持基础自噬很重要。然而,尽管网络适应足以维持基础自噬活性,但它们不能确保饥饿时自噬的诱导,其特征是脂质需求增加。因此,在这里,我们定义了RAB18在连接脂滴和自噬中的分子作用,强调了脂滴作为降解途径的脂源的重要性,并揭示了显着的自噬网络可塑性,包括磷酸化依赖性ATG9A活化,以补偿降低的脂质供应以拯救基础自噬活性。尽管网络适应足以维持基础自噬活性,但它们不能确保饥饿时自噬的诱导,其特征是脂质需求增加。因此,在这里,我们定义了RAB18在连接脂滴和自噬中的分子作用,强调了脂滴作为降解途径的脂源的重要性,并揭示了显着的自噬网络可塑性,包括磷酸化依赖性ATG9A活化,以补偿降低的脂质供应以拯救基础自噬活性。尽管网络适应足以维持基础自噬活性,但它们不能确保饥饿时自噬的诱导,其特征是脂质需求增加。因此,在这里,我们定义了RAB18在连接脂滴和自噬中的分子作用,强调了脂滴作为降解途径的脂源的重要性,并揭示了显着的自噬网络可塑性,包括磷酸化依赖性ATG9A活化,以补偿降低的脂质供应以拯救基础自噬活性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号