当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative stress-induced KLF4 activates inflammatory response through IL17RA and its downstream targets in retinal pigment epithelial cells.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.freeradbiomed.2019.12.029 Qian Sun 1 , Lili Gong 1 , Ruili Qi 1 , Wenjie Qing 1 , Ming Zou 1 , Qin Ke 1 , Lan Zhang 1 , Xiangcheng Tang 1 , Qian Nie 1 , Yuan Yang 1 , Andina Hu 1 , Xiaoyan Ding 1 , Lin Lu 1 , Yizhi Liu 1 , David Wan-Cheng Li 1
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.freeradbiomed.2019.12.029 Qian Sun 1 , Lili Gong 1 , Ruili Qi 1 , Wenjie Qing 1 , Ming Zou 1 , Qin Ke 1 , Lan Zhang 1 , Xiangcheng Tang 1 , Qian Nie 1 , Yuan Yang 1 , Andina Hu 1 , Xiaoyan Ding 1 , Lin Lu 1 , Yizhi Liu 1 , David Wan-Cheng Li 1
Affiliation
|
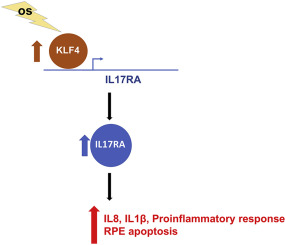
Age-related macular degeneration (AMD) is a leading cause of irreversible blindness worldwide. Oxidative stress (OS), inflammation and genetics are considered the key pathogenic factors contributing to AMD development. Recent evidence shows the pro-inflammatory interleukin 17 (IL17) signaling is activated in AMD patients and promotes disease pathogenesis. However, the interplay between OS and IL17 signaling, and the regulatory mechanism of IL17 pathway are largely unknown. OS-induced retinal pigment epithelial cell (RPE) damage causes both the initial pathogenesis of AMD and secondary degeneration of rods and cones. Healthy RPE is essential for ocular immune privilege, however, damaged RPE cells can activate inflammatory response. In the present study, we identified IL17RA, the principle receptor of IL17 signaling, is one of the most upregulated inflammatory genes in human RPE cells upon OS exposure. The prominent increase of IL17RA was also observed in RPE and retina of an AMD-like mouse model. Knockdown of IL17RA in RPE cells prevented OS-induced RPE cell apoptosis and reduced the inflammatory response in both RPE and macrophages. Furthermore, we found that transcription factor KLF4 directly activates IL17RA expression, therefore, promotes the production of IL1β and IL8 in an IL17RA-dependent manner. In addition, the mRNA level of KLF4 isoform 2 was positively correlated with that of IL17RA in AMD patients. Together, our study demonstrates an unrevealed relationship between IL17RA and OS, and a new regulatory mechanism of IL17RA by KLF4 in RPE cells. These findings suggest that inhibition of IL17RA as a new potential therapeutic target for AMD through RPE protection and inflammatory suppression upon OS exposure.
中文翻译:

氧化应激诱导的 KLF4 通过 IL17RA 及其在视网膜色素上皮细胞中的下游靶标激活炎症反应。
年龄相关性黄斑变性 (AMD) 是全球不可逆失明的主要原因。氧化应激 (OS)、炎症和遗传学被认为是导致 AMD 发展的关键致病因素。最近的证据表明,促炎白细胞介素 17 (IL17) 信号在 AMD 患者中被激活并促进疾病发病机制。然而,OS 和 IL17 信号之间的相互作用以及 IL17 通路的调节机制在很大程度上是未知的。OS 诱导的视网膜色素上皮细胞 (RPE) 损伤导致 AMD 的初始发病机制和视杆细胞和视锥细胞的继发性变性。健康的 RPE 对眼部免疫特权至关重要,然而,受损的 RPE 细胞会激活炎症反应。在本研究中,我们鉴定了 IL17RA,即 IL17 信号传导的主要受体,是 OS 暴露后人类 RPE 细胞中最上调的炎症基因之一。在 AMD 样小鼠模型的 RPE 和视网膜中也观察到 IL17RA 的显着增加。敲除 RPE 细胞中的 IL17RA 可防止 OS 诱导的 RPE 细胞凋亡并降低 RPE 和巨噬细胞的炎症反应。此外,我们发现转录因子 KLF4 直接激活 IL17RA 表达,因此以 IL17RA 依赖性方式促进 IL1β 和 IL8 的产生。此外,AMD 患者中 KLF4 亚型 2 的 mRNA 水平与 IL17RA 的 mRNA 水平呈正相关。总之,我们的研究证明了 IL17RA 和 OS 之间未揭示的关系,以及 KLF4 在 RPE 细胞中对 IL17RA 的新调节机制。
更新日期:2019-12-25
中文翻译:

氧化应激诱导的 KLF4 通过 IL17RA 及其在视网膜色素上皮细胞中的下游靶标激活炎症反应。
年龄相关性黄斑变性 (AMD) 是全球不可逆失明的主要原因。氧化应激 (OS)、炎症和遗传学被认为是导致 AMD 发展的关键致病因素。最近的证据表明,促炎白细胞介素 17 (IL17) 信号在 AMD 患者中被激活并促进疾病发病机制。然而,OS 和 IL17 信号之间的相互作用以及 IL17 通路的调节机制在很大程度上是未知的。OS 诱导的视网膜色素上皮细胞 (RPE) 损伤导致 AMD 的初始发病机制和视杆细胞和视锥细胞的继发性变性。健康的 RPE 对眼部免疫特权至关重要,然而,受损的 RPE 细胞会激活炎症反应。在本研究中,我们鉴定了 IL17RA,即 IL17 信号传导的主要受体,是 OS 暴露后人类 RPE 细胞中最上调的炎症基因之一。在 AMD 样小鼠模型的 RPE 和视网膜中也观察到 IL17RA 的显着增加。敲除 RPE 细胞中的 IL17RA 可防止 OS 诱导的 RPE 细胞凋亡并降低 RPE 和巨噬细胞的炎症反应。此外,我们发现转录因子 KLF4 直接激活 IL17RA 表达,因此以 IL17RA 依赖性方式促进 IL1β 和 IL8 的产生。此外,AMD 患者中 KLF4 亚型 2 的 mRNA 水平与 IL17RA 的 mRNA 水平呈正相关。总之,我们的研究证明了 IL17RA 和 OS 之间未揭示的关系,以及 KLF4 在 RPE 细胞中对 IL17RA 的新调节机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号