当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of Cutibacterium acnes with human bone marrow derived mesenchymal stem cells: a step toward understanding bone implant- associated infection development.
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.actbio.2019.12.028 M Dubus 1 , J Varin 2 , S Papa 2 , H Rammal 1 , J Chevrier 2 , E Maisonneuve 2 , C Mauprivez 3 , C Mongaret 4 , S C Gangloff 4 , F Reffuveille 4 , H Kerdjoudj 1
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.actbio.2019.12.028 M Dubus 1 , J Varin 2 , S Papa 2 , H Rammal 1 , J Chevrier 2 , E Maisonneuve 2 , C Mauprivez 3 , C Mongaret 4 , S C Gangloff 4 , F Reffuveille 4 , H Kerdjoudj 1
Affiliation
|
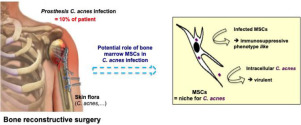
Crosstalk between mesenchymal stem cells (MSCs) and bacteria plays an important role in regulating the regenerative capacities of MSCs, fighting infections, modulating immune responses and maintaining tissue homeostasis. Commensal Cutibacterium acnes (C. acnes) bacterium becomes an opportunistic pathogen causing implant-associated infections. Herein, we examined MSCs/C. acnes interaction and analysed the subsequent bacteria and MSCs behaviours following infection. Human bone marrow derived MSCs were infected by two clinical and one laboratory C. acnes strains. Following 3h of interaction, all bacterial strains were able to invade MSCs. Viable intracellular bacteria acquired virulence factors by increasing biofilm formation and/or by affecting macrophage phagocytosis. Although the direct and indirect (through neutrophil stimulation) antibacterial effects of the MSCs secretome were not enhanced following C. acnes infection, ELISA analysis revealed that C. acnes clinical strains are able to license MSCs to become immunosuppressive cell-like by increasing the secretion of IL-6, IL-8, PGE-2, VEGF, TGF-β and HGF. Overall, these results showed a direct impact of C. acnes on bone marrow derived MSCs, providing new insights into the development of C. acnes during implant-associated infections. STATEMENT OF SIGNIFICANCE: The originality of this work relies on the study of relationship between human bone marrow derived mesenchymal stem cells (MSCs) phenotype and C. acnes clinical strains virulence following cell infection. Our major results showed that C. acnes are able to invade MSCs, inducing a transition of commensal to an opportunistic pathogen behaviour. Although the direct and indirect antibacterial effects were not enhanced following C. acnes infection, secretome analysis revealed that C. acnes clinical strains were able to license MSCs to become immunosuppressive and anti-fibrotic cell-like. These results showed a direct impact of C. acnes on bone marrow derived MSCs, providing new insights into the development of C. acnes during associated implant infections.
中文翻译:

痤疮角质杆菌与人骨髓来源的间充质干细胞的相互作用:迈向了解与骨植入物相关的感染发展的一步。
间充质干细胞(MSCs)与细菌之间的串扰在调节MSCs的再生能力,抵抗感染,调节免疫反应和维持组织动态平衡方面起着重要作用。痤疮丙酸杆菌(C. acnes)细菌成为机会病原体,引起植入物相关感染。在此,我们研究了MSC / C。痤疮相互作用,并分析了感染后随后的细菌和MSCs行为。人类骨髓来源的MSCs被两种临床和一种实验室的痤疮丙酸杆菌感染。相互作用3小时后,所有细菌菌株都能够入侵MSC。活细胞内细菌通过增加生物膜形成和/或通过影响巨噬细胞吞噬作用而获得毒力因子。尽管在痤疮丙酸杆菌感染后,MSCs分泌组的直接和间接(通过中性粒细胞刺激)抗菌作用并未得到增强,但ELISA分析显示,痤疮丙酸杆菌临床菌株能够通过增加MSCs的分泌而使MSC成为免疫抑制细胞样。 IL-6,IL-8,PGE-2,VEGF,TGF-β和HGF。总体而言,这些结果表明痤疮丙酸杆菌对骨髓来源的MSC具有直接影响,为植入相关感染期间痤疮丙酸杆菌的发展提供了新见解。意义声明:这项工作的原创性在于对人类骨髓源间充质干细胞(MSCs)表型与痤疮丙酸杆菌临床菌株毒力之间的关系的研究。我们的主要结果表明痤疮丙酸杆菌能够入侵MSC,引起共病向机会病原体行为的转变。尽管痤疮丙酸杆菌感染后没有直接和间接的抗菌作用,但分泌组分析显示,痤疮丙酸杆菌临床菌株能够许可MSC成为免疫抑制和抗纤维化细胞样。这些结果表明痤疮丙酸杆菌对源自骨髓的MSC具有直接影响,为相关的植入物感染期间痤疮丙酸杆菌的发展提供了新的见解。
更新日期:2019-12-25
中文翻译:

痤疮角质杆菌与人骨髓来源的间充质干细胞的相互作用:迈向了解与骨植入物相关的感染发展的一步。
间充质干细胞(MSCs)与细菌之间的串扰在调节MSCs的再生能力,抵抗感染,调节免疫反应和维持组织动态平衡方面起着重要作用。痤疮丙酸杆菌(C. acnes)细菌成为机会病原体,引起植入物相关感染。在此,我们研究了MSC / C。痤疮相互作用,并分析了感染后随后的细菌和MSCs行为。人类骨髓来源的MSCs被两种临床和一种实验室的痤疮丙酸杆菌感染。相互作用3小时后,所有细菌菌株都能够入侵MSC。活细胞内细菌通过增加生物膜形成和/或通过影响巨噬细胞吞噬作用而获得毒力因子。尽管在痤疮丙酸杆菌感染后,MSCs分泌组的直接和间接(通过中性粒细胞刺激)抗菌作用并未得到增强,但ELISA分析显示,痤疮丙酸杆菌临床菌株能够通过增加MSCs的分泌而使MSC成为免疫抑制细胞样。 IL-6,IL-8,PGE-2,VEGF,TGF-β和HGF。总体而言,这些结果表明痤疮丙酸杆菌对骨髓来源的MSC具有直接影响,为植入相关感染期间痤疮丙酸杆菌的发展提供了新见解。意义声明:这项工作的原创性在于对人类骨髓源间充质干细胞(MSCs)表型与痤疮丙酸杆菌临床菌株毒力之间的关系的研究。我们的主要结果表明痤疮丙酸杆菌能够入侵MSC,引起共病向机会病原体行为的转变。尽管痤疮丙酸杆菌感染后没有直接和间接的抗菌作用,但分泌组分析显示,痤疮丙酸杆菌临床菌株能够许可MSC成为免疫抑制和抗纤维化细胞样。这些结果表明痤疮丙酸杆菌对源自骨髓的MSC具有直接影响,为相关的植入物感染期间痤疮丙酸杆菌的发展提供了新的见解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号