当前位置:
X-MOL 学术
›
Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Periodic DFT study of water adsorption on m-WO3(001), m-WO3(100), h-WO3(001) and h-WO3(100). Role of hydroxyl groups on the stability of polar hexagonal surfaces.
Surface Science ( IF 2.1 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.susc.2019.121558 Oscar Hurtado-Aular , Alba B. Vidal , Aníbal Sierraalta , Rafael Añez
Surface Science ( IF 2.1 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.susc.2019.121558 Oscar Hurtado-Aular , Alba B. Vidal , Aníbal Sierraalta , Rafael Añez
|
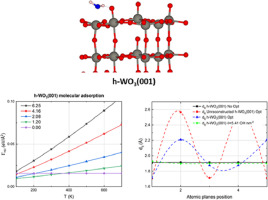
Abstract Water adsorption on the (001) and (100) surfaces of monoclinic and hexagonal WO3 was studied using a periodic DFT approach. The results showed that dissociation of water on these surfaces is less energetically favourable than molecular adsorption and such phenomenon is a consequence of surface deformations induced by OH groups. Water interacted stronger with the five-fold tungsten atoms of the hexagonal surfaces than with those atoms of the monoclinic surfaces. Nevertheless, adsorption energies are almost the same between both hexagonal and monoclinic surfaces when all available active surface sites were occupied by water molecules, that is, at the maximum water coverage. On monoclinic surfaces, water adsorption gradually became less stable as the coverage increased, but the opposite behaviour was observed with dissociative adsorption. On the (001) surfaces of the hexagonal structures, both molecular and dissociative adsorption became less stable as water coverage increased. On the other hand, on the (100) surface, dissociation was only observed at the maximum coverage. All surfaces remain hydrated at low temperatures; whereas at higher temperatures the most stable structures are the clean surfaces. Lastly, the formation of a monolayer of OH groups on hexagonal surfaces helps to avoid distortion, which provides an explanation of why hexagonal surfaces are observed experimentally despite of being intrinsically unstable.
中文翻译:

m-WO3(001)、m-WO3(100)、h-WO3(001)和h-WO3(100)上水吸附的周期性DFT研究。羟基对极性六边形表面稳定性的作用。
摘要 使用周期性 DFT 方法研究了单斜和六方 WO3 的 (001) 和 (100) 表面上的水吸附。结果表明,这些表面上的水离解在能量上不如分子吸附有利,这种现象是由 OH 基团引起的表面变形的结果。水与六边形表面的五重钨原子的相互作用比与单斜表面的那些原子的相互作用更强。然而,当所有可用的活性表面位点都被水分子占据时,即在最大水覆盖率下,六方和单斜表面之间的吸附能几乎相同。在单斜表面,随着覆盖率的增加,水吸附逐渐变得不稳定,但在解离吸附中观察到相反的行为。在六边形结构的(001)表面上,随着水覆盖率的增加,分子和解离吸附都变得不太稳定。另一方面,在 (100) 表面上,仅在最大覆盖率下观察到解离。所有表面在低温下保持水合;而在较高温度下,最稳定的结构是干净的表面。最后,在六边形表面上形成单层 OH 基团有助于避免变形,这解释了为什么尽管六边形表面本质上不稳定,但仍能通过实验观察到。所有表面在低温下保持水合;而在较高温度下,最稳定的结构是干净的表面。最后,在六边形表面上形成单层 OH 基团有助于避免变形,这解释了为什么尽管六边形表面本质上不稳定,但仍能通过实验观察到。所有表面在低温下保持水合;而在较高温度下,最稳定的结构是干净的表面。最后,在六边形表面上形成单层 OH 基团有助于避免变形,这解释了为什么尽管六边形表面本质上不稳定,但仍能通过实验观察到。
更新日期:2020-04-01
中文翻译:

m-WO3(001)、m-WO3(100)、h-WO3(001)和h-WO3(100)上水吸附的周期性DFT研究。羟基对极性六边形表面稳定性的作用。
摘要 使用周期性 DFT 方法研究了单斜和六方 WO3 的 (001) 和 (100) 表面上的水吸附。结果表明,这些表面上的水离解在能量上不如分子吸附有利,这种现象是由 OH 基团引起的表面变形的结果。水与六边形表面的五重钨原子的相互作用比与单斜表面的那些原子的相互作用更强。然而,当所有可用的活性表面位点都被水分子占据时,即在最大水覆盖率下,六方和单斜表面之间的吸附能几乎相同。在单斜表面,随着覆盖率的增加,水吸附逐渐变得不稳定,但在解离吸附中观察到相反的行为。在六边形结构的(001)表面上,随着水覆盖率的增加,分子和解离吸附都变得不太稳定。另一方面,在 (100) 表面上,仅在最大覆盖率下观察到解离。所有表面在低温下保持水合;而在较高温度下,最稳定的结构是干净的表面。最后,在六边形表面上形成单层 OH 基团有助于避免变形,这解释了为什么尽管六边形表面本质上不稳定,但仍能通过实验观察到。所有表面在低温下保持水合;而在较高温度下,最稳定的结构是干净的表面。最后,在六边形表面上形成单层 OH 基团有助于避免变形,这解释了为什么尽管六边形表面本质上不稳定,但仍能通过实验观察到。所有表面在低温下保持水合;而在较高温度下,最稳定的结构是干净的表面。最后,在六边形表面上形成单层 OH 基团有助于避免变形,这解释了为什么尽管六边形表面本质上不稳定,但仍能通过实验观察到。











































 京公网安备 11010802027423号
京公网安备 11010802027423号