当前位置:
X-MOL 学术
›
Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Reactivity, Selectivity and Structure of 2-butanol on Clean and Oxygen-covered Au/Pd(100) Alloys
Surface Science ( IF 2.1 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.susc.2019.121556 Zhenjun Li , Wilfred T. Tysoe
Surface Science ( IF 2.1 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.susc.2019.121556 Zhenjun Li , Wilfred T. Tysoe
|
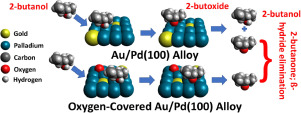
Abstract The surface chemistry of 2-butanol is studied on clean and oxygen-saturated Au/Pd(100) alloys using a combination of temperature-programmed desorption (TPD) and reflection-absorption infrared spectroscopy (RAIRS) as a function of alloy composition. The alloys are synthesized by evaporating gold on a Pd(100) substrate and heating to various temperatures to obtain a range of alloy compositions. It is found that 2-butanol initially forms 2-butoxide species both on clean and oxygen-saturated Au/Pd(100) alloys and can react either to reform 2-butanol or undergo a β-hydride elimination reaction to form 2-butanone. No 2-butanone is formed on alloys with palladium coverages below ~0.5 ML, and the 2-butanone yield increases linearly with palladium coverage at high coverages as the proportion of Pd‒Pd bridge sites on the alloy increases. Some ethylene is formed on alloys with palladium coverages between ~0.4 and 0.6 ML. Saturating the gold-palladium alloy with oxygen for palladium coverages above ~0.5 ML, where oxygen can dissociatively adsorb on the surface at pressures attainable in ultrahigh vacuum, increases the selectivity to 2-butanone formation to 100% for palladium coverages above ~0.75 ML, when Pd‒Pd bridge sites are present, while the selectivity decreases for palladium coverages of ~0.6 ML, when both isolated and bridge sites are present. This indicates that alloy catalysts containing just bridge sites at gold coverages below ~0.35 ML should be the most selective, while still being able to dissociatively adsorb oxygen
中文翻译:

清洁和氧包覆的 Au/Pd(100) 合金上 2-丁醇的反应性、选择性和结构
摘要 使用程序升温脱附 (TPD) 和反射-吸收红外光谱 (RAIRS) 的组合作为合金成分的函数,在清洁和氧饱和的 Au/Pd(100) 合金上研究 2-丁醇的表面化学。这些合金是通过在 Pd(100) 基材上蒸发金并加热到不同温度来合成的,以获得一系列合金成分。发现 2-丁醇最初在清洁和氧饱和的 Au/Pd(100) 合金上形成 2-丁醇物质,并且可以反应以重新形成 2-丁醇或进行 β-氢化物消除反应以形成 2-丁酮。钯覆盖率低于~0.5 ML 的合金上不会形成 2-丁酮,并且随着合金上 Pd-Pd 桥位比例的增加,2-丁酮产率随钯覆盖率在高覆盖率下线性增加。一些乙烯是在钯覆盖范围在 ~0.4 到 0.6 ML 之间的合金上形成的。用氧饱和金-钯合金以达到约 0.5 ML 以上的钯覆盖率,其中氧气可以在超高真空下可达到的压力下解离吸附在表面上,对于超过约 0.75 ML 的钯覆盖率,将形成 2-丁酮的选择性提高到 100%,当存在 Pd-Pd 桥位时,而当孤立位点和桥位都存在时,钯覆盖率约为 0.6 ML 时,选择性会降低。这表明仅包含金覆盖率低于 0.35 ML 的桥位的合金催化剂应该是最具选择性的,同时仍然能够解离吸附氧 5 ML,当存在 Pd-Pd 桥位时,氧可以在超高真空下可达到的压力下解离吸附在表面上,当钯覆盖率超过 0.75 ML 时,2-丁酮形成的选择性增加到 100%,而选择性降低当隔离位点和桥位点都存在时,钯覆盖率约为 0.6 ML。这表明仅包含金覆盖率低于 0.35 ML 的桥位的合金催化剂应该是最具选择性的,同时仍然能够解离吸附氧 5 ML,当存在 Pd-Pd 桥位时,氧可以在超高真空下可达到的压力下解离吸附在表面上,当钯覆盖率超过 0.75 ML 时,2-丁酮形成的选择性增加到 100%,而选择性降低当隔离位点和桥位点都存在时,钯覆盖率约为 0.6 ML。这表明仅包含金覆盖率低于 0.35 ML 的桥位的合金催化剂应该是最具选择性的,同时仍然能够解离吸附氧 当孤立站点和桥接站点都存在时。这表明仅包含金覆盖率低于 0.35 ML 的桥位的合金催化剂应该是最具选择性的,同时仍然能够解离吸附氧 当孤立站点和桥接站点都存在时。这表明仅包含金覆盖率低于 0.35 ML 的桥位的合金催化剂应该是最具选择性的,同时仍然能够解离吸附氧
更新日期:2020-04-01
中文翻译:

清洁和氧包覆的 Au/Pd(100) 合金上 2-丁醇的反应性、选择性和结构
摘要 使用程序升温脱附 (TPD) 和反射-吸收红外光谱 (RAIRS) 的组合作为合金成分的函数,在清洁和氧饱和的 Au/Pd(100) 合金上研究 2-丁醇的表面化学。这些合金是通过在 Pd(100) 基材上蒸发金并加热到不同温度来合成的,以获得一系列合金成分。发现 2-丁醇最初在清洁和氧饱和的 Au/Pd(100) 合金上形成 2-丁醇物质,并且可以反应以重新形成 2-丁醇或进行 β-氢化物消除反应以形成 2-丁酮。钯覆盖率低于~0.5 ML 的合金上不会形成 2-丁酮,并且随着合金上 Pd-Pd 桥位比例的增加,2-丁酮产率随钯覆盖率在高覆盖率下线性增加。一些乙烯是在钯覆盖范围在 ~0.4 到 0.6 ML 之间的合金上形成的。用氧饱和金-钯合金以达到约 0.5 ML 以上的钯覆盖率,其中氧气可以在超高真空下可达到的压力下解离吸附在表面上,对于超过约 0.75 ML 的钯覆盖率,将形成 2-丁酮的选择性提高到 100%,当存在 Pd-Pd 桥位时,而当孤立位点和桥位都存在时,钯覆盖率约为 0.6 ML 时,选择性会降低。这表明仅包含金覆盖率低于 0.35 ML 的桥位的合金催化剂应该是最具选择性的,同时仍然能够解离吸附氧 5 ML,当存在 Pd-Pd 桥位时,氧可以在超高真空下可达到的压力下解离吸附在表面上,当钯覆盖率超过 0.75 ML 时,2-丁酮形成的选择性增加到 100%,而选择性降低当隔离位点和桥位点都存在时,钯覆盖率约为 0.6 ML。这表明仅包含金覆盖率低于 0.35 ML 的桥位的合金催化剂应该是最具选择性的,同时仍然能够解离吸附氧 5 ML,当存在 Pd-Pd 桥位时,氧可以在超高真空下可达到的压力下解离吸附在表面上,当钯覆盖率超过 0.75 ML 时,2-丁酮形成的选择性增加到 100%,而选择性降低当隔离位点和桥位点都存在时,钯覆盖率约为 0.6 ML。这表明仅包含金覆盖率低于 0.35 ML 的桥位的合金催化剂应该是最具选择性的,同时仍然能够解离吸附氧 当孤立站点和桥接站点都存在时。这表明仅包含金覆盖率低于 0.35 ML 的桥位的合金催化剂应该是最具选择性的,同时仍然能够解离吸附氧 当孤立站点和桥接站点都存在时。这表明仅包含金覆盖率低于 0.35 ML 的桥位的合金催化剂应该是最具选择性的,同时仍然能够解离吸附氧











































 京公网安备 11010802027423号
京公网安备 11010802027423号