当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing the mechanism of protein-lipid interactions for a putative membrane curvature sensor in plant endoplasmic reticulum.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.bbamem.2019.183160 Rhiannon L Brooks 1 , Ann M Dixon 2
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.bbamem.2019.183160 Rhiannon L Brooks 1 , Ann M Dixon 2
Affiliation
|
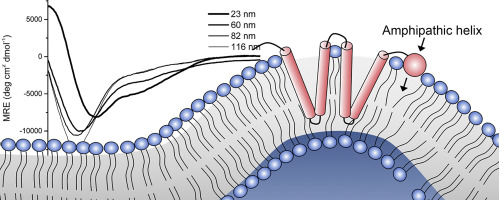
Membrane curvature sensing via helical protein domains, such as those identified in Amphiphysin and ArfGAP1, have been linked to a diverse range of cellular processes. However, these regions can vary significantly between different protein families and thus remain challenging to identify from sequence alone. Greater insight into the protein-lipid interactions that drive this behavior could lead to production of therapeutics that specifically target highly curved membranes. Here we demonstrate the curvature-dependence of membrane binding for an amphipathic helix (APH) in a plant reticulon, namely RTNLB13 from A. thaliana. We utilize solution-state nuclear magnetic resonance spectroscopy to establish the exact location of the APH and map the residues involved in protein-membrane interactions at atomic resolution. We find that the hydrophobic residues making up the membrane binding site are conserved throughout all A. thaliana reticulons. Our results also provide mechanistic insight that leads us to propose that membrane binding by this APH may act as a feedback element, only forming when ER tubules reach a critical size and adding stabilization to these structures without disrupting the bilayer. A shallow hydrophobic binding interface appears to be a feature shared more broadly across helical curvature sensors and would automatically restrict the penetration depth of these structures into the membrane. We also suggest this APH is highly tuned to the composition of the membrane in which it resides, and that this property may be universal in curvature sensors thus rationalizing the variety of mechanisms reported for these functional elements.
中文翻译:

揭示了植物内质网中假定的膜曲率传感器的蛋白质-脂质相互作用机制。
通过螺旋蛋白结构域的膜曲率感测(例如在Amphiphysin和ArfGAP1中鉴定的那些结构域)已与多种细胞过程相关联。但是,这些区域在不同的蛋白质家族之间可能会有很大差异,因此仍然难以从单独的序列中进行鉴定。对驱动这种行为的蛋白质-脂质相互作用的更深入了解可能会导致产生专门针对高度弯曲的膜的疗法。在这里,我们证明了植物网状结构中的两亲性螺旋(APH)膜结合的曲率依赖性,即来自拟南芥的RTNLB13。我们利用溶液状态核磁共振波谱确定APH的确切位置,并以原子分辨率绘制参与蛋白质-膜相互作用的残基图。我们发现,组成膜结合位点的疏水残基在所有拟南芥网状结构中都保守。我们的研究结果还提供了机制上的见解,使我们提出这种APH的膜结合可以充当反馈元素,仅在ER小管达到临界大小时形成,并在不破坏双层的情况下为这些结构增加稳定性。浅的疏水性结合界面似乎是螺旋曲率传感器之间更广泛共享的特征,并且会自动限制这些结构进入膜的穿透深度。我们还建议该APH高度适合其所驻留的膜的组成,并且该特性在曲率传感器中可能是通用的,因此可以合理化为这些功能元件报告的各种机理。
更新日期:2019-12-25
中文翻译:

揭示了植物内质网中假定的膜曲率传感器的蛋白质-脂质相互作用机制。
通过螺旋蛋白结构域的膜曲率感测(例如在Amphiphysin和ArfGAP1中鉴定的那些结构域)已与多种细胞过程相关联。但是,这些区域在不同的蛋白质家族之间可能会有很大差异,因此仍然难以从单独的序列中进行鉴定。对驱动这种行为的蛋白质-脂质相互作用的更深入了解可能会导致产生专门针对高度弯曲的膜的疗法。在这里,我们证明了植物网状结构中的两亲性螺旋(APH)膜结合的曲率依赖性,即来自拟南芥的RTNLB13。我们利用溶液状态核磁共振波谱确定APH的确切位置,并以原子分辨率绘制参与蛋白质-膜相互作用的残基图。我们发现,组成膜结合位点的疏水残基在所有拟南芥网状结构中都保守。我们的研究结果还提供了机制上的见解,使我们提出这种APH的膜结合可以充当反馈元素,仅在ER小管达到临界大小时形成,并在不破坏双层的情况下为这些结构增加稳定性。浅的疏水性结合界面似乎是螺旋曲率传感器之间更广泛共享的特征,并且会自动限制这些结构进入膜的穿透深度。我们还建议该APH高度适合其所驻留的膜的组成,并且该特性在曲率传感器中可能是通用的,因此可以合理化为这些功能元件报告的各种机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号