Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acetylation of SUMO1 Alters Interactions with the SIMs of PML and Daxx in a Protein-Specific Manner.
Structure ( IF 4.4 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.str.2019.11.019 Xavier H Mascle 1 , Christina Gagnon 1 , Haytham M Wahba 2 , Mathieu Lussier-Price 1 , Laurent Cappadocia 1 , Kazuyasu Sakaguchi 3 , James G Omichinski 1
Structure ( IF 4.4 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.str.2019.11.019 Xavier H Mascle 1 , Christina Gagnon 1 , Haytham M Wahba 2 , Mathieu Lussier-Price 1 , Laurent Cappadocia 1 , Kazuyasu Sakaguchi 3 , James G Omichinski 1
Affiliation
|
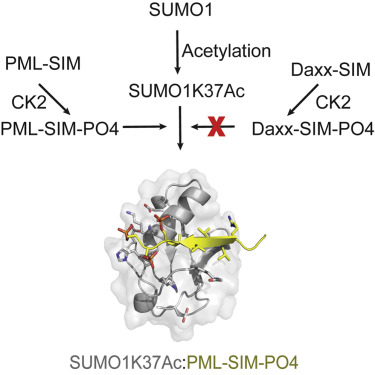
The interactions between SUMO proteins and SUMO-interacting motif (SIM) in nuclear bodies formed by the promyelocytic leukemia (PML) protein (PML-NBs) have been shown to be modulated by either phosphorylation of the SIMs or acetylation of SUMO proteins. However, little is known about how this occurs at the atomic level. In this work, we examined the role that acetylation of SUMO1 plays on its binding to the phosphorylated SIMs (phosphoSIMs) of PML and Daxx. Our results demonstrate that SUMO1 binding to the phosphoSIM of either PML or Daxx is dramatically reduced by acetylation at either K39 or K46. However, acetylation at K37 only impacts binding to Daxx. Structures of acetylated SUMO1 variants bound to the phosphoSIMs of PML and Daxx demonstrate that there is structural plasticity in SUMO-SIM interactions. The plasticity observed in these structures provides a robust mechanism for regulating SUMO-SIM interactions in PML-NBs using signaling generated post-translational modifications.
中文翻译:

SUMO1的乙酰化以蛋白质特异性方式改变与PML和Daxx SIM的相互作用。
SUMO蛋白与由早幼粒细胞白血病(PML)蛋白(PML-NBs)形成的核体中的SUMO相互作用基序(SIM)之间的相互作用已显示受SIMS的磷酸化或SUMO蛋白的乙酰化作用的调节。但是,对于这种现象在原子水平上是如何发生的则知之甚少。在这项工作中,我们研究了SUMO1的乙酰化在其与PML和Daxx的磷酸化SIM(phosphoSIMs)结合上的作用。我们的结果表明,SUMO1与PML或Daxx的phosphoSIM的结合通过在K39或K46处的乙酰化作用而大大降低。但是,K37处的乙酰化只会影响与Daxx的结合。绑定到PML和Daxx的phosphoSIMs的乙酰化SUMO1变体的结构表明SUMO-SIM相互作用中存在结构可塑性。
更新日期:2019-12-25
中文翻译:

SUMO1的乙酰化以蛋白质特异性方式改变与PML和Daxx SIM的相互作用。
SUMO蛋白与由早幼粒细胞白血病(PML)蛋白(PML-NBs)形成的核体中的SUMO相互作用基序(SIM)之间的相互作用已显示受SIMS的磷酸化或SUMO蛋白的乙酰化作用的调节。但是,对于这种现象在原子水平上是如何发生的则知之甚少。在这项工作中,我们研究了SUMO1的乙酰化在其与PML和Daxx的磷酸化SIM(phosphoSIMs)结合上的作用。我们的结果表明,SUMO1与PML或Daxx的phosphoSIM的结合通过在K39或K46处的乙酰化作用而大大降低。但是,K37处的乙酰化只会影响与Daxx的结合。绑定到PML和Daxx的phosphoSIMs的乙酰化SUMO1变体的结构表明SUMO-SIM相互作用中存在结构可塑性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号