当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-pot synthesis and molecular docking of some new spiropyranindol-2-one derivatives as immunomodulatory agents and in vitro antimicrobial potential with DNA gyrase inhibitor.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.ejmech.2019.111977 Mohamed A Salem 1 , Ahmed Ragab 2 , Ahmed A Askar 3 , Abeer El-Khalafawy 4 , Abeer H Makhlouf 5
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.ejmech.2019.111977 Mohamed A Salem 1 , Ahmed Ragab 2 , Ahmed A Askar 3 , Abeer El-Khalafawy 4 , Abeer H Makhlouf 5
Affiliation
|
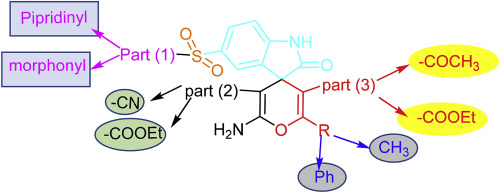
a series of 2-oxospiro[indoline-3,4'-pyran]derivatives 4 and 7 were obtained in good yield under mild conditions from the one-pot reaction of indole-2,3-dione derivatives 1, appropriate methylene active nitriles 2 and β-dicarbonyl compound 3 or 6. The newly synthesized compounds were characterized and evaluated for their in vitro antibacterial, antifungal as well as immunomodulatory activity. According to MIC values, the most potent compounds 4f, 4h, 7a, 7c, 7e, 7f, 7g, 8a, and 8c were evaluated for MBC and displayed high activity to killing pathogens with a good MBC value against norfloxacin as well as investigated against an extended panel of multidrug resistance bacteria (MDRB) and exhibited promising to moderate multidrug resistance activities, compounds 7f showed the much better than norfloxacin with higher potency results. Furthermore, the most potent compounds showed an increase in the intracellular killing activity of neutrophils which confirmed the immunostimulatory power. Eight of the nine active compounds exhibited inhibitory activities with IC50 ranged between (18.07 ± 0.18) to (27.03 ± 0.24) μM stronger than ciprofloxacin (26.43 ± 0.64 μM) for S. aureus DNA gyrase. Molecular docking was performed inside the active site of S. aureus DNA gyrase to predict the binding mode.
中文翻译:

一锅合成和一些新的螺吡喃并吲哚-2-酮衍生物作为免疫调节剂的分子对接和DNA促旋酶抑制剂的体外抗菌潜力。
从吲哚-2,3-二酮衍生物1,合适的亚甲基活性腈2的单锅反应中,在温和条件下以高收率获得了一系列2-oxospiro [indoline-3,4'-pyran]衍生物4和7。以及β-二羰基化合物3或6。对新合成的化合物进行了表征,并对其体外抗菌,抗真菌和免疫调节活性进行了评估。根据MIC值,评估了最有效的化合物4f,4h,7a,7c,7e,7f,7g,8a和8c的MBC并显示出很高的杀灭病原体活性,对诺氟沙星具有良好的MBC值,并对其进行了研究扩展的多药耐药菌(MDRB)面板并显示出有希望的中度多药耐药活性,化合物7f的效果比诺氟沙星好得多,并具有更高的效价结果。此外,最有效的化合物显示嗜中性粒细胞的细胞内杀伤活性增加,这证实了其免疫刺激能力。9种活性化合物中的8种对金黄色葡萄球菌DNA促旋酶的抑制活性比环丙沙星(26.43±0.64μM)强(IC.50)在(18.07±0.18)至(27.03±0.24)μM之间。在金黄色葡萄球菌DNA促旋酶的活性位点内进行分子对接以预测结合模式。
更新日期:2019-12-25
中文翻译:

一锅合成和一些新的螺吡喃并吲哚-2-酮衍生物作为免疫调节剂的分子对接和DNA促旋酶抑制剂的体外抗菌潜力。
从吲哚-2,3-二酮衍生物1,合适的亚甲基活性腈2的单锅反应中,在温和条件下以高收率获得了一系列2-oxospiro [indoline-3,4'-pyran]衍生物4和7。以及β-二羰基化合物3或6。对新合成的化合物进行了表征,并对其体外抗菌,抗真菌和免疫调节活性进行了评估。根据MIC值,评估了最有效的化合物4f,4h,7a,7c,7e,7f,7g,8a和8c的MBC并显示出很高的杀灭病原体活性,对诺氟沙星具有良好的MBC值,并对其进行了研究扩展的多药耐药菌(MDRB)面板并显示出有希望的中度多药耐药活性,化合物7f的效果比诺氟沙星好得多,并具有更高的效价结果。此外,最有效的化合物显示嗜中性粒细胞的细胞内杀伤活性增加,这证实了其免疫刺激能力。9种活性化合物中的8种对金黄色葡萄球菌DNA促旋酶的抑制活性比环丙沙星(26.43±0.64μM)强(IC.50)在(18.07±0.18)至(27.03±0.24)μM之间。在金黄色葡萄球菌DNA促旋酶的活性位点内进行分子对接以预测结合模式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号