当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel pleconaril derivatives: Influence of substituents in the isoxazole and phenyl rings on the antiviral activity against enteroviruses.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.ejmech.2019.112007 Anna Egorova 1 , Elena Kazakova 1 , Birgit Jahn 2 , Sean Ekins 3 , Vadim Makarov 1 , Michaela Schmidtke 2
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.ejmech.2019.112007 Anna Egorova 1 , Elena Kazakova 1 , Birgit Jahn 2 , Sean Ekins 3 , Vadim Makarov 1 , Michaela Schmidtke 2
Affiliation
|
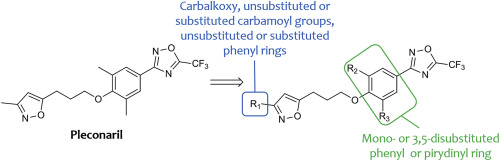
Today, there are no medicines to treat enterovirus and rhinovirus infections. In the present study, a series of novel pleconaril derivatives with substitutions in the isoxazole and phenyl rings was synthesized and evaluated for their antiviral activity against a panel of pleconaril-sensitive and -resistant enteroviruses. Studies of the structure-activity relationship demonstrate the crucial role of the N,N-dimethylcarbamoyl group in the isoxazole ring for antiviral activity against pleconaril-resistant viruses. In addition, one or two substituents in the phenyl ring directly impact on the spectrum of antienteroviral activity. The 3-(3-methyl-4-(3-(3-N,N-dimethylcarbamoyl-isoxazol-5-yl)propoxy)phenyl)-5-trifluoromethyl-1,2,4-oxadiazole 10g was among the compounds exhibiting the strongest activity against pleconaril-resistant as well as pleconaril-susceptible enteroviruses with IC50 values from 0.02 to 5.25 μM in this series. Compound 10g demonstrated markedly less CYP3A4 induction than pleconaril, was non-mutagenic, and was bioavailable after intragastric administration in mice. These results highlight compound 10g as a promising potential candidate as a broad spectrum enterovirus and rhinovirus inhibitor for further preclinical investigations.
中文翻译:

新型pleconaril衍生物:异恶唑和苯环中的取代基对肠病毒的抗病毒活性的影响。
如今,没有药物可治疗肠病毒和鼻病毒感染。在本研究中,合成了一系列在异恶唑和苯环上具有取代基的新型pleconaril衍生物,并评估了它们对一组pleconaril敏感和耐药肠病毒的抗病毒活性。结构活性关系的研究表明,异恶唑环中的N,N-二甲基氨基甲酰基对抗pleconaril的病毒具有抗病毒活性。另外,苯环中的一个或两个取代基直接影响抗肠病毒活性的光谱。3-(3-甲基-4-(3-(3-N,N-二甲基氨基甲酰基-异恶唑-5-基)丙氧基)苯基)-5-三氟甲基-1,2,10克4-恶二唑是表现出最强抗pleconaril耐药性和pleconaril易感性肠病毒活性的化合物,在该系列中IC50值为0.02至5.25μM。化合物10g的CYP3A4诱导作用比普乐那利明显少,是非诱变的,在小鼠胃内给药后可被生物利用。这些结果突出了化合物10g作为广谱肠病毒和鼻病毒抑制剂有希望的潜在候选物,用于进一步的临床前研究。
更新日期:2019-12-25
中文翻译:

新型pleconaril衍生物:异恶唑和苯环中的取代基对肠病毒的抗病毒活性的影响。
如今,没有药物可治疗肠病毒和鼻病毒感染。在本研究中,合成了一系列在异恶唑和苯环上具有取代基的新型pleconaril衍生物,并评估了它们对一组pleconaril敏感和耐药肠病毒的抗病毒活性。结构活性关系的研究表明,异恶唑环中的N,N-二甲基氨基甲酰基对抗pleconaril的病毒具有抗病毒活性。另外,苯环中的一个或两个取代基直接影响抗肠病毒活性的光谱。3-(3-甲基-4-(3-(3-N,N-二甲基氨基甲酰基-异恶唑-5-基)丙氧基)苯基)-5-三氟甲基-1,2,10克4-恶二唑是表现出最强抗pleconaril耐药性和pleconaril易感性肠病毒活性的化合物,在该系列中IC50值为0.02至5.25μM。化合物10g的CYP3A4诱导作用比普乐那利明显少,是非诱变的,在小鼠胃内给药后可被生物利用。这些结果突出了化合物10g作为广谱肠病毒和鼻病毒抑制剂有希望的潜在候选物,用于进一步的临床前研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号