当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimisation of estrogen receptor subtype-selectivity of a 4-Aryl-4H-chromene scaffold previously identified by virtual screening.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.bmc.2019.115261 Miriam Carr 1 , Andrew J S Knox 2 , Daniel K Nevin 3 , Niamh O'Boyle 4 , Shu Wang 4 , Billy Egan 4 , Thomas McCabe 5 , Brendan Twamley 5 , Daniela M Zisterer 3 , David G Lloyd 3 , Mary J Meegan 4
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.bmc.2019.115261 Miriam Carr 1 , Andrew J S Knox 2 , Daniel K Nevin 3 , Niamh O'Boyle 4 , Shu Wang 4 , Billy Egan 4 , Thomas McCabe 5 , Brendan Twamley 5 , Daniela M Zisterer 3 , David G Lloyd 3 , Mary J Meegan 4
Affiliation
|
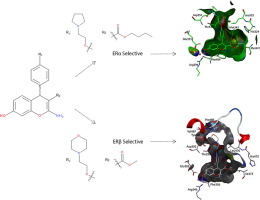
4-Aryl-4H-Chromene derivatives have been previously shown to exhibit anti-proliferative, apoptotic and anti-angiogenic activity in a variety of tumor models in vitro and in vivo generally via activation of caspases through inhibition of tubulin polymerisation. We have previously identified by Virtual Screening (VS) a 4-aryl-4H-chromene scaffold, of which two examples were shown to bind Estrogen Receptor α and β with low nanomolar affinity and <20-fold selectivity for α over β and low micromolar anti-proliferative activity in the MCF-7 cell line. Thus, using the 4-aryl-4H-chromene scaffold as a starting point, a series of compounds with a range of basic arylethers at C-4 and modifications at the C3-ester substituent of the benzopyran ring were synthesised, producing some potent ER antagonists in the MCF-7 cell line which were highly selective for ERα (compound 35; 350-fold selectivity) or ERβ (compound 42; 170-fold selectivity).
中文翻译:

以前通过虚拟筛选确定的4-Aryl-4H-苯甲基支架的雌激素受体亚型选择性的优化。
先前已证明4-芳基-4H-Chromene衍生物在体外和体内的各种肿瘤模型中通常通过抑制微管蛋白聚合来激活胱天蛋白酶来表现出抗增殖,凋亡和抗血管生成活性。我们先前已经通过虚拟筛选(VS)鉴定了4-芳基-4H-亚甲基支架,其中两个实例显示以较低的纳摩尔亲和力结合雌激素受体α和β,且相对于β和低微摩尔而言,α的选择性<20倍MCF-7细胞系中的抗增殖活性。因此,以4-芳基-4H-亚甲基支架为起点,合成了一系列在C-4处具有一系列碱性芳基醚并在苯并吡喃环的C3-酯取代基处进行了修饰的化合物,
更新日期:2019-12-25
中文翻译:

以前通过虚拟筛选确定的4-Aryl-4H-苯甲基支架的雌激素受体亚型选择性的优化。
先前已证明4-芳基-4H-Chromene衍生物在体外和体内的各种肿瘤模型中通常通过抑制微管蛋白聚合来激活胱天蛋白酶来表现出抗增殖,凋亡和抗血管生成活性。我们先前已经通过虚拟筛选(VS)鉴定了4-芳基-4H-亚甲基支架,其中两个实例显示以较低的纳摩尔亲和力结合雌激素受体α和β,且相对于β和低微摩尔而言,α的选择性<20倍MCF-7细胞系中的抗增殖活性。因此,以4-芳基-4H-亚甲基支架为起点,合成了一系列在C-4处具有一系列碱性芳基醚并在苯并吡喃环的C3-酯取代基处进行了修饰的化合物,











































 京公网安备 11010802027423号
京公网安备 11010802027423号