当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile synthesis of 1,3,4-oxadiazoles via iodine promoted oxidative annulation of methyl-azaheteroarenes and hydrazides
Tetrahedron ( IF 2.1 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.tet.2019.130887 Zhi-Hao Shang , Ji-Na Sun , Jiang-Shan Guo , Yuan-Yuan Sun , Wei-Zhao Weng , Zhen-Xiao Zhang , Zeng-Jing Li , Yan-Ping Zhu
中文翻译:

通过碘轻松合成1,3,4-恶二唑,促进了甲基氮杂叠氮芳烃和酰肼的氧化环化反应
更新日期:2019-12-25
Tetrahedron ( IF 2.1 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.tet.2019.130887 Zhi-Hao Shang , Ji-Na Sun , Jiang-Shan Guo , Yuan-Yuan Sun , Wei-Zhao Weng , Zhen-Xiao Zhang , Zeng-Jing Li , Yan-Ping Zhu
|
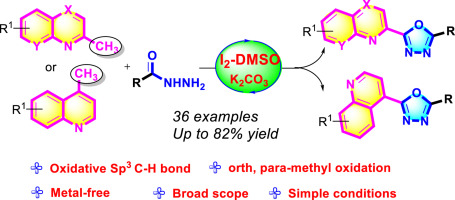
An oxidative sp3 C–H bond of methyl-azaheteroarenes protocol was reported for the synthesis of 1,3,4-oxadiazoles via [4 + 1] annulation with hydrazides. This protocol enables 1,3,4-oxadiazole and quinoline linked diheterocycles via selective oxidation of sp3 C–H bond of methyl-azaheteroarenes in the presence of I2-DMSO. The reaction has a broad substrate scope and good functional group tolerance for methyl-azaheteroarenes and hydrazides.
中文翻译:

通过碘轻松合成1,3,4-恶二唑,促进了甲基氮杂叠氮芳烃和酰肼的氧化环化反应
据报道,甲基[azazeteroarenes]协议的氧化sp 3 C–H键可通过[4 +1]酰肼环合反应合成1,3,4-恶二唑。该方案通过在I 2 -DMSO存在下对甲基-氮杂杂环芳烃的sp 3 C–H键进行选择性氧化来实现1,3,4-恶二唑和喹啉连接的二杂环。该反应具有广泛的底物范围,并且对甲基-氮杂环杂芳烃和酰肼具有良好的官能团耐受性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号