Tetrahedron ( IF 2.1 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.tet.2019.130888 Shintarou Ogawa , Kengo Miyata , Sho Kawakami , Shinji Tanaka , Masato Kitamura
|
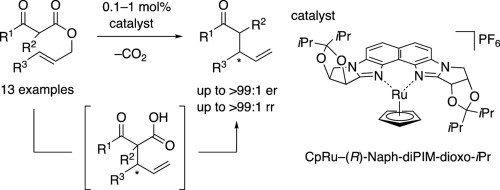
Carroll-rearrangement-type intramolecular decarboxylative allylation of β-keto allyl esters with a cationic CpRu complex bearing a chiral bisamidine-type bidentate ligand is reported. The catalytic system allows the efficient transformation of the mono substituted, 3-arylallyl esters of β-keto acids to the corresponding γ,δ-enones. Notably, the catalytic turnover is more than an order higher than that of the previously reported examples. The developed reaction system displays improved regioselectivity and enantioselectivity and a broad substrate scope. Mechanistic studies using NMR experiments, substrate-structure/reactivity relationship studies, and cross-over experiment reveal a possible reaction pathway involving the enolate of the β-keto acid.
中文翻译:

CpRu II-手性双am配合物催化β-酮烯丙基酯的不对称卡罗尔型脱羧烯丙基化
报道了β-酮烯丙基酯与带有手性双am型二齿配体的阳离子CpRu配合物的卡洛尔重排型分子内脱羧烯丙基化。该催化体系允许将β-酮酸的单取代的3-芳基烯丙基酯有效地转化为相应的γ,δ-烯酮。明显地,催化转化率比先前报道的实例的催化转化率高一个数量级。发达的反应系统显示出改进的区域选择性和对映选择性以及广泛的底物范围。使用NMR实验的机理研究,底物结构/反应性关系研究和交叉实验揭示了可能的反应途径,其中涉及β-酮酸的烯醇化物。









































 京公网安备 11010802027423号
京公网安备 11010802027423号