当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NHC-Pd(II)-Azole Complexes Catalyzed Suzuki−Miyaura Cross-Coupling of Sterically Hindered Aryl Chlorides with Arylboronic Acids
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.tetlet.2019.151541 Yingying Zhang , Rong Zhang , Chang Ni , Xue Zhang , Yanji Li , Qingwen Lu , Yuxuan Zhao , Fangwai Han , Yongfei Zeng , Guiyan Liu
中文翻译:

NHC-Pd(II)-氮杂配合物催化立体受阻的芳基氯化物与芳基硼酸的Suzuki-Miyaura交叉偶联
更新日期:2019-12-25
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.tetlet.2019.151541 Yingying Zhang , Rong Zhang , Chang Ni , Xue Zhang , Yanji Li , Qingwen Lu , Yuxuan Zhao , Fangwai Han , Yongfei Zeng , Guiyan Liu
|
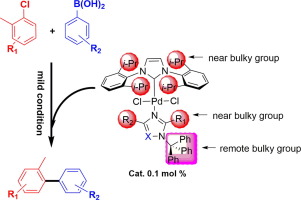
In order to synthesize hindered biaryls efficiently, a series of NHC-Pd(II)-azole complexes bearing sterically hindered ligands were synthesized and characterized. The steric environment effect as well as the electronic effect of the azole ligands has been assessed. All these complexes were applied in the Suzuki−Miyaura cross-coupling reaction of sterically hindered aryl chlorides with low catalysts loadings (0.1 mol %) under mild conditions in air and good to excellent isolated yields of sterically hindered biaryls were obtained.
中文翻译:

NHC-Pd(II)-氮杂配合物催化立体受阻的芳基氯化物与芳基硼酸的Suzuki-Miyaura交叉偶联
为了有效地合成受阻联芳基,合成并表征了一系列带有空间受阻配体的NHC-Pd(II)-唑配合物。已经评估了空间环境效应以及唑配体的电子效应。所有这些络合物均在空气中温和条件下,以低催化剂负载量(0.1摩尔%)用于空间受阻芳基氯的Suzuki-Miyaura交叉偶联反应中,并获得良好至优异的空间受阻联芳基分离产率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号