Particuology ( IF 4.1 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.partic.2019.10.006 Menghua Tian , Jianwei Guo , Zhi Wang , Jianwei Cao , Xuzhong Gong
|
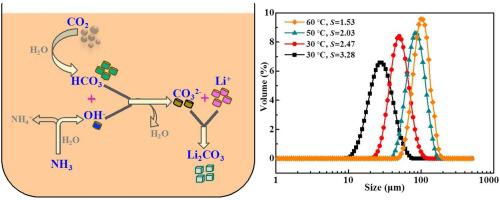
In this study, the gas‒liquid reactive crystallization of LiCl–NH3·H2O–CO2 was adopted to produce Li2CO3. The weakly alkaline nature of NH3·H2O in the absence of any recarbonation process resulted in a unimodal and easily controllable particle size distribution (PSD) of the obtained Li2CO3. The reaction temperature significantly influenced both the Li2CO3 particle size and PSD. By increasing the temperature from 25 to 60 °C, the volume weighted mean particle size increased from 50.5 to 100.5 μm, respectively. The Li2CO3 secondary nucleation rate and growth rate were obtained by focused beam reflectance measurements and a laser particle size analyzer, respectively. The secondary nucleation rate of Li2CO3 reduced as a function of temperature, whereas the growth rate increased. In addition to improving the surface energy of the crystals to enhance the growth process, higher temperatures also reduced the supersolubility of Li2CO3, which also plays a role to decrease the secondary nucleation rate. At a constant temperature, supersaturation affects the Li2CO3 particle size through the synergistic effect of secondary nucleation and growth. Hence, with improved supersaturation, the mean particle size of Li2CO3 decreased. The results provide a meaningful way to evaluate the crystallization process and to regulate the particle size.
中文翻译:

LiCl–NH 3 ·H 2 O–CO 2气液反应结晶中二次成核和生长对碳酸锂粒度的协同效应
本研究采用LiCl–NH 3 ·H 2 O–CO 2的气液反应结晶法制备Li 2 CO 3。在没有任何再碳酸化过程的情况下,NH 3 ·H 2 O的弱碱性导致所获得的Li 2 CO 3的单峰且易于控制的粒度分布(PSD)。反应温度显着影响Li 2 CO 3粒径和PSD。通过将温度从25℃提高到60℃,体积加权平均粒径分别从50.5μm增加到100.5μm。Li 2 CO通过聚焦光束反射率测量和激光粒度分析仪分别获得3次晶核形成速率和生长速率。Li 2 CO 3的二次成核速率随温度降低,而生长速率增加。高温除了改善晶体的表面能以增强生长过程外,还降低了Li 2 CO 3的超溶解度,这也起到降低二次成核率的作用。在恒定温度下,过饱和会影响Li 2 CO 3通过二次成核和生长的协同作用获得的颗粒尺寸。因此,随着过饱和度的提高,Li 2 CO 3的平均粒径减小。结果为评估结晶过程和调节粒径提供了有意义的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号