Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
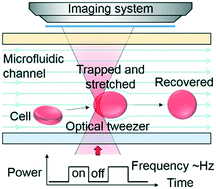
An optofluidic "tweeze-and-drag" cell stretcher in a microfluidic channel.
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-01-07 , DOI: 10.1039/c9lc01026b Zhanshi Yao 1 , Ching Chi Kwan 1 , Andrew W Poon 1
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-01-07 , DOI: 10.1039/c9lc01026b Zhanshi Yao 1 , Ching Chi Kwan 1 , Andrew W Poon 1
Affiliation
|
The mechanical properties of biological cells are utilized as an inherent, label-free biomarker to indicate physiological and pathological changes of cells. Although various optical and microfluidic techniques have been developed for cell mechanical characterization, there is still a strong demand for non-contact and continuous methods. Here, by combining optical and microfluidic techniques in a single desktop platform, we demonstrate an optofluidic cell stretcher based on a "tweeze-and-drag" mechanism using a periodically chopped, tightly focused laser beam as an optical tweezer to trap a cell temporarily and a flow-induced drag force to stretch the cell in a microfluidic channel transverse to the tweezer. Our method leverages the advantages of non-contact optical forces and a microfluidic flow for both cell stretching and continuous cell delivery. We demonstrate the stretcher for mechanical characterization of rabbit red blood cells (RBCs), with a throughput of ∼1 cell per s at a flow rate of 2.5 μl h-1 at a continuous-wave laser power of ∼25 mW at a wavelength of 1064 nm (chopped at 2 Hz). We estimate the spring constant of RBCs to be ∼14.9 μN m-1. Using the stretcher, we distinguish healthy RBCs and RBCs treated with glutaraldehyde at concentrations of 5 × 10-4% to 2.5 × 10-3%, with a strain-to-concentration sensitivity of ∼-1529. By increasing the optical power to ∼45 mW, we demonstrate cell-stretching under a higher flow rate of 4 μl h-1, with a higher throughput of ∼1.5 cells per s and a higher sensitivity of ∼-2457. Our technique shows promise for applications in the fields of healthcare monitoring and biomechanical studies.
中文翻译:

在微流体通道中的光流体“镊子并拖动”细胞担架。
生物细胞的机械特性被用作固有的,无标记的生物标记,以指示细胞的生理和病理变化。尽管已开发出各种光学和微流体技术来表征细胞的机械特性,但对非接触和连续方法的需求仍然很高。在这里,通过在单个台式机平台上结合光学和微流体技术,我们演示了一种基于“镊子并拖拉”机制的光流体细胞担架,该机制使用周期性切碎的紧密聚焦的激光束作为光学镊子来暂时捕获细胞并流动引起的拖曳力使细胞在横向于镊子的微流体通道中拉伸。我们的方法利用了非接触式光学力和微流体流的优势,可进行细胞拉伸和连续细胞递送。我们展示了用于表征兔红细胞(RBCs)的担架,在2.5μlh-1的流速下,在〜25 mW的连续波激光功率下,其吞吐能力为〜1个细胞/秒。 1064 nm(以2 Hz斩波)。我们估计RBC的弹簧常数约为14.9μNm-1。使用担架,我们可以区分健康的红细胞和用戊二醛浓度在5×10-4%到2.5×10-3%的戊二醛处理的菌株,其对浓度的敏感度约为-1529。通过将光功率增加到〜45 mW,我们证明了在4μlh-1的更高流速下的细胞拉伸,〜1.5细胞/ s的更高通量和〜-2457的更高灵敏度。
更新日期:2020-02-13
中文翻译:

在微流体通道中的光流体“镊子并拖动”细胞担架。
生物细胞的机械特性被用作固有的,无标记的生物标记,以指示细胞的生理和病理变化。尽管已开发出各种光学和微流体技术来表征细胞的机械特性,但对非接触和连续方法的需求仍然很高。在这里,通过在单个台式机平台上结合光学和微流体技术,我们演示了一种基于“镊子并拖拉”机制的光流体细胞担架,该机制使用周期性切碎的紧密聚焦的激光束作为光学镊子来暂时捕获细胞并流动引起的拖曳力使细胞在横向于镊子的微流体通道中拉伸。我们的方法利用了非接触式光学力和微流体流的优势,可进行细胞拉伸和连续细胞递送。我们展示了用于表征兔红细胞(RBCs)的担架,在2.5μlh-1的流速下,在〜25 mW的连续波激光功率下,其吞吐能力为〜1个细胞/秒。 1064 nm(以2 Hz斩波)。我们估计RBC的弹簧常数约为14.9μNm-1。使用担架,我们可以区分健康的红细胞和用戊二醛浓度在5×10-4%到2.5×10-3%的戊二醛处理的菌株,其对浓度的敏感度约为-1529。通过将光功率增加到〜45 mW,我们证明了在4μlh-1的更高流速下的细胞拉伸,〜1.5细胞/ s的更高通量和〜-2457的更高灵敏度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号