当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of Np(v) with borate in alkaline, dilute-to-concentrated, NaCl and MgCl2 solutions.
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-01-14 , DOI: 10.1039/c9dt04430b K Hinz 1 , D Fellhauer , X Gaona , M Vespa , K Dardenne , D Schild , T Yokosawa , M A Silver , D T Reed , T E Albrecht-Schmitt , M Altmaier , H Geckeis
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-01-14 , DOI: 10.1039/c9dt04430b K Hinz 1 , D Fellhauer , X Gaona , M Vespa , K Dardenne , D Schild , T Yokosawa , M A Silver , D T Reed , T E Albrecht-Schmitt , M Altmaier , H Geckeis
Affiliation
|
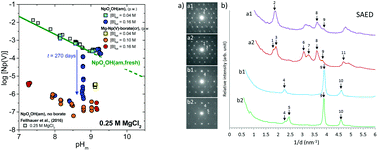
The interaction of Np(v) with borate was investigated in 0.1-5.0 M NaCl and 0.25-4.5 M MgCl2 solutions with 7.2 ≤ pHm ≤ 10.0 (pHm = -log[H+]) and 0.004 M ≤ [B]tot ≤ 0.16 M. Experiments were performed under an Ar-atmosphere at T = (22 ± 2) °C using a combination of under- and oversaturation solubility experiments, NIR spectroscopy, and extensive solid phase characterization. A bathochromic shift (≈5 nm) in the Np(v) band at λ = 980 nm indicates the formation of weak Np(v)-borate complexes under mildly alkaline pHm-conditions. The identification of an isosbestic point supports the formation of a single Np(v)-borate species in dilute MgCl2 systems, whereas a more complex aqueous speciation (eventually involving the formation of several Np(v)-borate species) is observed in concentrated MgCl2 solutions. The solubility of freshly prepared NpO2OH(am) remained largely unaltered in NaCl and MgCl2 solutions with [B]tot = 0.04 M within the timeframe of this study (t ≤ 300 days). At [B]tot = 0.16 M, a kinetically hindered but very significant drop in the solubility of Np(v) (3-4 log10-units, compared to borate-free systems) was observed in NaCl and dilute MgCl2 solutions with pHm ≤ 9. The drop in the solubility was accompanied by a clear change in the colour of the solid phase (from green to white-greyish). XRD and TEM analyses showed that the amorphous NpO2OH(am) "starting material" transformed into crystalline solid phases with similar XRD patterns in NaCl and MgCl2 systems. XPS, SEM-EDS and EXAFS further indicated that borate and Na/Mg participate stoichiometrically in the formation of such solid phases. Additional undersaturation solubility experiments using the newly formed Na-Np(v)-borate(cr) and Mg-Np(v)-borate(cr) compounds further confirmed the low solubility ([Np(v)]aq ≈ 10-6-10-7 M) of such solid phases in mildly alkaline pHm-conditions. The formation of these solid phases represents a previously unreported retention mechanism for the highly mobile Np(v) under boundary conditions (pHm, [B]tot, ionic strength) of relevance to certain repository concepts for nuclear waste disposal.
中文翻译:

Np(v)与硼酸盐在碱性,稀浓缩至NaCl和MgCl2溶液中的相互作用。
在7.2≤pHm≤10.0(pHm = -log [H +])和0.004 M≤[B] tot≤0.16 M的0.1-5.0 M NaCl和0.25-4.5 M MgCl2溶液中研究了Np(v)与硼酸盐的相互作用实验是在氩气中于T =(22±2)°C下进行的,结合了欠饱和和过饱和溶解度实验,NIR光谱和广泛的固相表征。Np(v)谱带在λ= 980 nm处发生红移(≈5nm),表明在弱碱性pHm条件下形成了弱Np(v)-硼酸盐络合物。等渗点的鉴定支持在稀MgCl2系统中形成单个Np(v)-硼酸盐物种,而在浓MgCl2中观察到更复杂的水形态(最终涉及多个Np(v)-硼酸盐物种的形成)。解决方案。在本研究的时间范围内(t≤300天),在[B] tot = 0.04 M的NaCl和MgCl2溶液中,新制备的NpO2OH(am)的溶解度仍然保持不变。在[B] tot = 0.16 M时,在NaCl和pHm≤的MgCl2稀溶液中观察到了Np(v)的动力学受阻但非常显着的下降(与无硼酸盐体系相比为3-4 log10单位)。 9.溶解度下降伴随着固相颜色的明显变化(从绿色变为白色灰色)。XRD和TEM分析表明,在NaCl和MgCl2系统中,无定形NpO2OH(am)“起始材料”转变为具有相似XRD图案的结晶固相。XPS,SEM-EDS和EXAFS进一步表明,硼酸盐和Na / Mg在化学计量上参与了此类固相的形成。使用新形成的Na-Np(v)-硼酸盐(cr)和Mg-Np(v)-硼酸盐(cr)化合物进行的其他不饱和溶解度实验进一步证实了低溶解度([Np(v)] aq≈10-6- 10-7 M)的此类固相在弱碱性pHm条件下。这些固相的形成代表了在与某些处置核废料处置库概念有关的边界条件(pHm,[B] tot,离子强度)的条件下,高度可移动的Np(v)的先前未报道的保留机制。
更新日期:2020-02-10
中文翻译:

Np(v)与硼酸盐在碱性,稀浓缩至NaCl和MgCl2溶液中的相互作用。
在7.2≤pHm≤10.0(pHm = -log [H +])和0.004 M≤[B] tot≤0.16 M的0.1-5.0 M NaCl和0.25-4.5 M MgCl2溶液中研究了Np(v)与硼酸盐的相互作用实验是在氩气中于T =(22±2)°C下进行的,结合了欠饱和和过饱和溶解度实验,NIR光谱和广泛的固相表征。Np(v)谱带在λ= 980 nm处发生红移(≈5nm),表明在弱碱性pHm条件下形成了弱Np(v)-硼酸盐络合物。等渗点的鉴定支持在稀MgCl2系统中形成单个Np(v)-硼酸盐物种,而在浓MgCl2中观察到更复杂的水形态(最终涉及多个Np(v)-硼酸盐物种的形成)。解决方案。在本研究的时间范围内(t≤300天),在[B] tot = 0.04 M的NaCl和MgCl2溶液中,新制备的NpO2OH(am)的溶解度仍然保持不变。在[B] tot = 0.16 M时,在NaCl和pHm≤的MgCl2稀溶液中观察到了Np(v)的动力学受阻但非常显着的下降(与无硼酸盐体系相比为3-4 log10单位)。 9.溶解度下降伴随着固相颜色的明显变化(从绿色变为白色灰色)。XRD和TEM分析表明,在NaCl和MgCl2系统中,无定形NpO2OH(am)“起始材料”转变为具有相似XRD图案的结晶固相。XPS,SEM-EDS和EXAFS进一步表明,硼酸盐和Na / Mg在化学计量上参与了此类固相的形成。使用新形成的Na-Np(v)-硼酸盐(cr)和Mg-Np(v)-硼酸盐(cr)化合物进行的其他不饱和溶解度实验进一步证实了低溶解度([Np(v)] aq≈10-6- 10-7 M)的此类固相在弱碱性pHm条件下。这些固相的形成代表了在与某些处置核废料处置库概念有关的边界条件(pHm,[B] tot,离子强度)的条件下,高度可移动的Np(v)的先前未报道的保留机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号