当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A copper-clad lithiophilic current collector for dendrite-free lithium metal anodes†
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2019-12-24 , DOI: 10.1039/c9ta11237e Ke Chen 1, 2, 3, 4, 5 , Rajesh Pathak 1, 2, 3, 4, 5 , Ashim Gurung 1, 2, 3, 4, 5 , Khan M. Reza 1, 2, 3, 4, 5 , Nabin Ghimire 1, 2, 3, 4, 5 , Jyotshna Pokharel 1, 2, 3, 4, 5 , Abiral Baniya 1, 2, 3, 4, 5 , Wei He 1, 2, 3, 4, 5 , James J. Wu 5, 6, 7 , Qiquan (Quinn) Qiao 1, 2, 3, 4, 5 , Yue Zhou 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2019-12-24 , DOI: 10.1039/c9ta11237e Ke Chen 1, 2, 3, 4, 5 , Rajesh Pathak 1, 2, 3, 4, 5 , Ashim Gurung 1, 2, 3, 4, 5 , Khan M. Reza 1, 2, 3, 4, 5 , Nabin Ghimire 1, 2, 3, 4, 5 , Jyotshna Pokharel 1, 2, 3, 4, 5 , Abiral Baniya 1, 2, 3, 4, 5 , Wei He 1, 2, 3, 4, 5 , James J. Wu 5, 6, 7 , Qiquan (Quinn) Qiao 1, 2, 3, 4, 5 , Yue Zhou 1, 2, 3, 4, 5
Affiliation
|
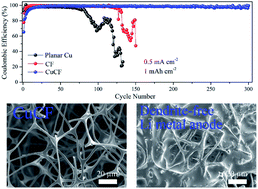
Lithium (Li) metal has been considered as one of the most attractive anode materials of Li batteries due to its high theoretical capacity and low electrochemical potential. However, dendrite formation and large volume change during battery operation hinder its commercialization. Here, we created a three-dimensional (3D) light-weight and mechanically flexible copper-clad carbon framework (CuCF) as a lithiophilic current collector. The CuCF can be made by scalable pyrolysis of a melamine–formaldehyde foam (MF) followed by copper electroplating. The carbon framework (CF) without copper cladding has a lower conductivity (4.32 × 10−4 S cm−1) and fewer non-uniform lithium nucleation sites, leading to lithium dendrite growth during plating/stripping. By surface engineering with copper-cladding, the CuCF has a much higher conductivity (1.38 × 10−2 S cm−1) and more Li nucleation sites which allow a uniform and smooth Li deposition. Moreover, the excellent mechanical flexibility and enlarged surface area of the CuCF current collector can accommodate volume expansion and reduce local current density. As a result, a dendrite-free Li metal anode is achieved with a high coulombic efficiency of 99.5% even after 300 plating/stripping cycles (∼1200 hours). Significantly, it can last for more than 170 cycles at a high current of 5 mA cm−2 in a symmetric cell cycling test. Furthermore, a Li/lithium iron phosphate (LFP) cell exhibits a long cycling life at a high current of 1C.
中文翻译:

用于无枝晶状锂金属阳极的覆铜耐油集电器†
锂(Li)金属由于其高理论容量和低电化学势而被认为是锂电池最有吸引力的负极材料之一。然而,在电池操作期间枝晶的形成和大的体积变化阻碍了其商业化。在这里,我们创建了三维(3D)轻巧且机械柔韧性的覆铜碳骨架(CuCF)作为亲硫的集电器。CuCF可以通过三聚氰胺-甲醛泡沫(MF)的可扩展热解,然后进行铜电镀来制备。没有覆铜的碳骨架(CF)的电导率较低(4.32×10 -4 S cm -1)和更少的不均匀锂成核位点,从而导致锂枝晶在镀覆/剥离过程中生长。通过采用覆铜的表面工程技术,CuCF具有更高的电导率(1.38×10 -2 S cm -1)和更多的锂成核位点,从而可以实现均匀且平滑的锂沉积。而且,CuCF集电器的优异的机械柔韧性和增大的表面积可适应体积膨胀并降低局部电流密度。结果,即使经过300次电镀/剥离循环(〜1200小时),仍可实现99.5%的高库仑效率的无枝晶锂金属阳极。值得注意的是,在5 mA cm -2的高电流下,它可以持续超过170个循环在对称细胞周期测试中。此外,锂/磷酸铁锂(LFP)电池在1C的高电流下显示出长循环寿命。
更新日期:2020-01-06
中文翻译:

用于无枝晶状锂金属阳极的覆铜耐油集电器†
锂(Li)金属由于其高理论容量和低电化学势而被认为是锂电池最有吸引力的负极材料之一。然而,在电池操作期间枝晶的形成和大的体积变化阻碍了其商业化。在这里,我们创建了三维(3D)轻巧且机械柔韧性的覆铜碳骨架(CuCF)作为亲硫的集电器。CuCF可以通过三聚氰胺-甲醛泡沫(MF)的可扩展热解,然后进行铜电镀来制备。没有覆铜的碳骨架(CF)的电导率较低(4.32×10 -4 S cm -1)和更少的不均匀锂成核位点,从而导致锂枝晶在镀覆/剥离过程中生长。通过采用覆铜的表面工程技术,CuCF具有更高的电导率(1.38×10 -2 S cm -1)和更多的锂成核位点,从而可以实现均匀且平滑的锂沉积。而且,CuCF集电器的优异的机械柔韧性和增大的表面积可适应体积膨胀并降低局部电流密度。结果,即使经过300次电镀/剥离循环(〜1200小时),仍可实现99.5%的高库仑效率的无枝晶锂金属阳极。值得注意的是,在5 mA cm -2的高电流下,它可以持续超过170个循环在对称细胞周期测试中。此外,锂/磷酸铁锂(LFP)电池在1C的高电流下显示出长循环寿命。











































 京公网安备 11010802027423号
京公网安备 11010802027423号