当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, docking and biological evaluation of chalcones as promising antidiabetic agents.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.bioorg.2019.103527 Aluru Rammohan 1 , Baki Vijaya Bhaskar 2 , Nagam Venkateswarlu 3 , Wei Gu 4 , Grigory V Zyryanov 5
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.bioorg.2019.103527 Aluru Rammohan 1 , Baki Vijaya Bhaskar 2 , Nagam Venkateswarlu 3 , Wei Gu 4 , Grigory V Zyryanov 5
Affiliation
|
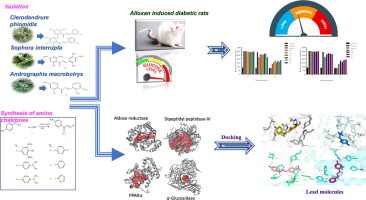
Diabetes mellitus (DM) is a serious chronic metabolic disorder which occurs due to dysfunction of insulin and therapeutic approaches are poor. It is an under estimation that 387 million people currently suffering globally with diabetic and more than 592 million people may be affected by 2030. It makes an urgent necessity to discover novel drugs to control amplified diabetic populations. In this study, amino chalcones (3a-j) were synthesized and hydroxy chalcones (3g-j) were isolated from natural source such as Sophora interrupta, Clerodendrum phlomidis and Andrographis macrobotrys. Structural elucidation was carried out using Mass, 1H and 13C NMR Spectra. In vivo studies were carried out with alloxan induced diabetic rats (100 mg/kg) which reveals compounds 3c, 3a and 3h have significant antidiabetic efficacy with decreased blood glucose levels in the diabetic rats while compared with control rats. Besides, docking studies with aldose reductase, dipeptidyl peptidase, PPAR and glucosidase were monitored which accomplishes that the compounds 3c, 3i, 3a and 3d have eloquent binding affinity (kcal/mol) with aldose reductase, besides the chalcones 3c, 3b, 3d, 3e and 3i were also showed inhibition with DPP-IV, PPAR-α and α-glucosidase. Also, these compounds explicated distinct interactions i.e., π-π, π-cationic, polar, electrostatic and hydrophobic bonds were observed with key residues of binding pockets. Bioavailability is disclosed with Lipinski rule of five and the design pharmacokinetic as well as pharmacodynamic properties are reliable. Therefore, chalcones were implied as antidiabetic leads for in further studies and could be worthwhile for the development of new classes of effective antidiabetic agents.
中文翻译:

查耳酮作为有前途的抗糖尿病药物的设计,合成,对接和生物学评估。
糖尿病(DM)是一种严重的慢性代谢性疾病,由于胰岛素功能障碍而发生,治疗方法较差。据估计,到2030年,全球目前将有3.87亿糖尿病患者和超过5.92亿人受到影响。迫切需要发现新药来控制糖尿病人群的增长。在这项研究中,合成了氨基查耳酮(3a-j),并从自然来源中分离了羟基查耳酮(3g-j),例如苦豆,香附子和穿心莲。使用质谱,1 H和13 C NMR光谱进行结构阐明。用四氧嘧啶诱导的糖尿病大鼠(100 mg / kg)进行了体内研究,结果显示化合物3c,与对照大鼠相比,糖尿病大鼠中的3a和3h具有显着的抗糖尿病功效,其血糖水平降低。此外,还监测了与醛糖还原酶,二肽基肽酶,PPAR和葡萄糖苷酶的对接研究,结果表明,除了查耳酮3c,3b,3d, 3e和3i也显示出被DPP-IV,PPAR-α和α-葡萄糖苷酶抑制。而且,这些化合物表现出独特的相互作用,即在结合口袋的关键残基上观察到了π-π,π-阳离子,极性,静电和疏水键。根据5的Lipinski规则公开了生物利用度,并且设计药代动力学和药效学性质是可靠的。所以,
更新日期:2019-12-25
中文翻译:

查耳酮作为有前途的抗糖尿病药物的设计,合成,对接和生物学评估。
糖尿病(DM)是一种严重的慢性代谢性疾病,由于胰岛素功能障碍而发生,治疗方法较差。据估计,到2030年,全球目前将有3.87亿糖尿病患者和超过5.92亿人受到影响。迫切需要发现新药来控制糖尿病人群的增长。在这项研究中,合成了氨基查耳酮(3a-j),并从自然来源中分离了羟基查耳酮(3g-j),例如苦豆,香附子和穿心莲。使用质谱,1 H和13 C NMR光谱进行结构阐明。用四氧嘧啶诱导的糖尿病大鼠(100 mg / kg)进行了体内研究,结果显示化合物3c,与对照大鼠相比,糖尿病大鼠中的3a和3h具有显着的抗糖尿病功效,其血糖水平降低。此外,还监测了与醛糖还原酶,二肽基肽酶,PPAR和葡萄糖苷酶的对接研究,结果表明,除了查耳酮3c,3b,3d, 3e和3i也显示出被DPP-IV,PPAR-α和α-葡萄糖苷酶抑制。而且,这些化合物表现出独特的相互作用,即在结合口袋的关键残基上观察到了π-π,π-阳离子,极性,静电和疏水键。根据5的Lipinski规则公开了生物利用度,并且设计药代动力学和药效学性质是可靠的。所以,











































 京公网安备 11010802027423号
京公网安备 11010802027423号