当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and synthesis of novel cylopentapyrazoles bearing 1,2,3-thiadiazole moiety as potent antifungal agents.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.bioorg.2019.103509 Betül Giray 1 , Ayşe Esra Karadağ 2 , Özgecan Şavluğ İpek 3 , Hanife Pekel 4 , Mustafa Güzel 5 , Hatice Başpınar Küçük 6
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-12-24 , DOI: 10.1016/j.bioorg.2019.103509 Betül Giray 1 , Ayşe Esra Karadağ 2 , Özgecan Şavluğ İpek 3 , Hanife Pekel 4 , Mustafa Güzel 5 , Hatice Başpınar Küçük 6
Affiliation
|
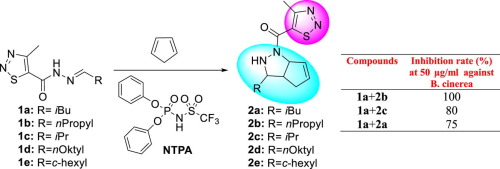
In drug-resistant phytopathogenic fungi, there has been extensive research on microbiological and antifungal drug development. In this study, a novel series of cylopentapyrazole bearing a 1,2,3-thiadiazole ring 2a-e were designed and synthesized according to the principle of combination of bioactive structures. Thus, we have employed a [3 + 2] cycloaddition with 4-methyl-[1,2,3] thiadiazole-5-carboxylic acid hydrazones 1a-e and cyclopentadiene ring. Novel synthesized compounds were identified with IR, 1H and 13C NMR, mass spectrometry and elemental analysis then, antifungal activities were assayed. Based on our study, a combination of the compounds 1a and 2b possess remarkable antifungal activity against Botrytis cinerea AHU 9424 with 100% inhibition. EC50 values were calculated by studying different doses in combinations with high inhibition rates. The combination of 1a + 2b has an EC50 value at 6.37 and 13.85 µg/ml concentrations against B. cinerea and F. culmorum, respectively. The combination of compound 1a + 2b, having a cylopentapyrazole ring on the 1,2,3-thiadiazole backbone, shows promising fungicidal activity and deserves further development. Additionally, the homology model of the CYP51 enzyme that belongs toFusarium moniliformewas generated using CYP51B (PDB ID: 6CR2), and molecular docking was performed using this homology model for each compound. The results of this study clearly indicate that these novel compounds can be identified as promising lead compounds and potential fungicidal agents in future.
中文翻译:

设计和合成带有1,2,3-噻二唑部分的新型环戊吡唑类作为有效的抗真菌剂。
在耐药的植物病原真菌中,对微生物和抗真菌药物的开发进行了广泛的研究。在这项研究中,根据生物活性结构的组合原理,设计并合成了一系列带有1,2,3-噻二唑环2a-e的环戊吡唑。因此,我们使用了4-甲基-[1,2,3]噻二唑-5-羧酸carboxylic1a-e和环戊二烯环的[3 + 2]环加成反应。通过IR,1H和13C NMR,质谱和元素分析鉴定了新合成的化合物,然后测定了抗真菌活性。根据我们的研究,化合物1a和2b的组合对灰葡萄孢AHU 9424具有显着的抗真菌活性,且具有100%的抑制作用。通过研究具有高抑制率的不同剂量来计算EC50值。1a + 2b的组合分别对灰葡萄孢和枯草镰刀菌的EC50值分别为6.37和13.85 µg / ml。在1,2,3-噻二唑骨架上具有环戊吡唑环的化合物1a + 2b的组合显示出有希望的杀真菌活性,值得进一步开发。另外,属于使用CYP51B产生的单核镰孢(Fusarium moniliformewas)的CYP51酶的同源性模型(PDB ID:6CR2),并且使用该同源性模型对每种化合物进行分子对接。这项研究的结果清楚地表明,这些新型化合物可以在未来被鉴定为有前途的先导化合物和潜在的杀真菌剂。分别对灰葡萄孢和枯萎镰刀菌的浓度为85 µg / ml。在1,2,3-噻二唑骨架上具有环戊吡唑环的化合物1a + 2b的组合显示出有希望的杀真菌活性,值得进一步开发。另外,属于使用CYP51B产生的单核镰孢(Fusarium moniliformewas)的CYP51酶的同源性模型(PDB ID:6CR2),并且使用该同源性模型对每种化合物进行分子对接。这项研究的结果清楚地表明,这些新型化合物可以在未来被鉴定为有前途的先导化合物和潜在的杀真菌剂。分别对灰葡萄孢和枯萎镰刀菌的浓度为85 µg / ml。在1,2,3-噻二唑骨架上具有环戊吡唑环的化合物1a + 2b的组合显示出有希望的杀真菌活性,值得进一步开发。另外,属于使用CYP51B产生的单核镰孢(Fusarium moniliformewas)的CYP51酶的同源性模型(PDB ID:6CR2),并且使用该同源性模型对每种化合物进行分子对接。这项研究的结果清楚地表明,这些新型化合物可以在未来被鉴定为有前途的先导化合物和潜在的杀真菌剂。使用CYP51B(PDB ID:6CR2)生成的属于镰孢镰刀菌(Fusarium moniliformewas)的CYP51酶的同源模型(PDB ID:6CR2),并使用该同源模型对每种化合物进行分子对接。这项研究的结果清楚地表明,这些新型化合物可以在未来被鉴定为有前途的先导化合物和潜在的杀真菌剂。使用CYP51B(PDB ID:6CR2)生成的属于镰孢镰刀菌(Fusarium moniliformewas)的CYP51酶的同源模型(PDB ID:6CR2),并使用该同源模型对每种化合物进行分子对接。这项研究的结果清楚地表明,这些新型化合物可以在未来被鉴定为有前途的先导化合物和潜在的杀真菌剂。
更新日期:2019-12-25
中文翻译:

设计和合成带有1,2,3-噻二唑部分的新型环戊吡唑类作为有效的抗真菌剂。
在耐药的植物病原真菌中,对微生物和抗真菌药物的开发进行了广泛的研究。在这项研究中,根据生物活性结构的组合原理,设计并合成了一系列带有1,2,3-噻二唑环2a-e的环戊吡唑。因此,我们使用了4-甲基-[1,2,3]噻二唑-5-羧酸carboxylic1a-e和环戊二烯环的[3 + 2]环加成反应。通过IR,1H和13C NMR,质谱和元素分析鉴定了新合成的化合物,然后测定了抗真菌活性。根据我们的研究,化合物1a和2b的组合对灰葡萄孢AHU 9424具有显着的抗真菌活性,且具有100%的抑制作用。通过研究具有高抑制率的不同剂量来计算EC50值。1a + 2b的组合分别对灰葡萄孢和枯草镰刀菌的EC50值分别为6.37和13.85 µg / ml。在1,2,3-噻二唑骨架上具有环戊吡唑环的化合物1a + 2b的组合显示出有希望的杀真菌活性,值得进一步开发。另外,属于使用CYP51B产生的单核镰孢(Fusarium moniliformewas)的CYP51酶的同源性模型(PDB ID:6CR2),并且使用该同源性模型对每种化合物进行分子对接。这项研究的结果清楚地表明,这些新型化合物可以在未来被鉴定为有前途的先导化合物和潜在的杀真菌剂。分别对灰葡萄孢和枯萎镰刀菌的浓度为85 µg / ml。在1,2,3-噻二唑骨架上具有环戊吡唑环的化合物1a + 2b的组合显示出有希望的杀真菌活性,值得进一步开发。另外,属于使用CYP51B产生的单核镰孢(Fusarium moniliformewas)的CYP51酶的同源性模型(PDB ID:6CR2),并且使用该同源性模型对每种化合物进行分子对接。这项研究的结果清楚地表明,这些新型化合物可以在未来被鉴定为有前途的先导化合物和潜在的杀真菌剂。分别对灰葡萄孢和枯萎镰刀菌的浓度为85 µg / ml。在1,2,3-噻二唑骨架上具有环戊吡唑环的化合物1a + 2b的组合显示出有希望的杀真菌活性,值得进一步开发。另外,属于使用CYP51B产生的单核镰孢(Fusarium moniliformewas)的CYP51酶的同源性模型(PDB ID:6CR2),并且使用该同源性模型对每种化合物进行分子对接。这项研究的结果清楚地表明,这些新型化合物可以在未来被鉴定为有前途的先导化合物和潜在的杀真菌剂。使用CYP51B(PDB ID:6CR2)生成的属于镰孢镰刀菌(Fusarium moniliformewas)的CYP51酶的同源模型(PDB ID:6CR2),并使用该同源模型对每种化合物进行分子对接。这项研究的结果清楚地表明,这些新型化合物可以在未来被鉴定为有前途的先导化合物和潜在的杀真菌剂。使用CYP51B(PDB ID:6CR2)生成的属于镰孢镰刀菌(Fusarium moniliformewas)的CYP51酶的同源模型(PDB ID:6CR2),并使用该同源模型对每种化合物进行分子对接。这项研究的结果清楚地表明,这些新型化合物可以在未来被鉴定为有前途的先导化合物和潜在的杀真菌剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号