当前位置:
X-MOL 学术
›
Nat. Struct. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coupled structural transitions enable highly cooperative regulation of human CTPS2 filaments.
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2019-12-23 , DOI: 10.1038/s41594-019-0352-5 Eric M Lynch 1 , Justin M Kollman 1
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2019-12-23 , DOI: 10.1038/s41594-019-0352-5 Eric M Lynch 1 , Justin M Kollman 1
Affiliation
|
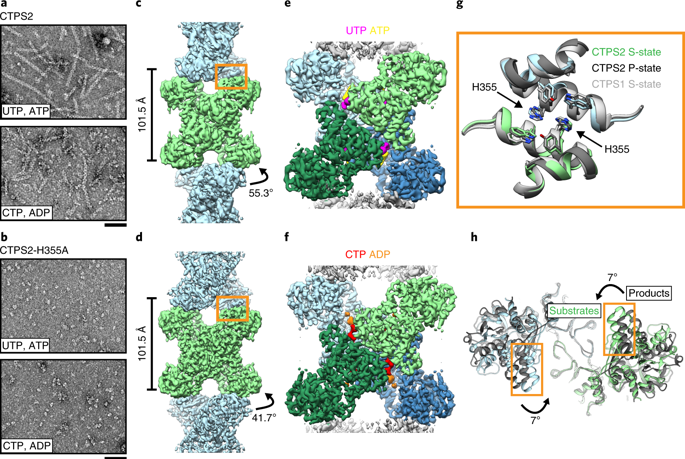
Many enzymes assemble into defined oligomers, providing a mechanism for cooperatively regulating activity. Recent studies have described a mode of regulation in which enzyme activity is modulated by polymerization into large-scale filaments. Here we describe an ultrasensitive form of polymerization-based regulation employed by human CTP synthase 2 (CTPS2). Cryo-EM structures reveal that CTPS2 filaments dynamically switch between active and inactive forms in response to changes in substrate and product levels. Linking the conformational state of many CTPS2 subunits in a filament results in highly cooperative regulation, greatly exceeding the limits of cooperativity for the CTPS2 tetramer alone. The structures reveal a link between conformation and control of ammonia channeling between the enzyme's active sites, and explain differences in regulation of human CTPS isoforms. This filament-based mechanism of enhanced cooperativity demonstrates how the widespread phenomenon of enzyme polymerization can be adapted to achieve different regulatory outcomes.
中文翻译:

耦合结构转变能够高度协同调节人类 CTPS2 细丝。
许多酶组装成特定的寡聚体,提供了一种协同调节活性的机制。最近的研究描述了一种调节模式,其中酶活性通过聚合成大规模细丝来调节。在这里,我们描述了人类 CTP 合酶 2 (CTPS2) 采用的一种基于聚合的超灵敏调节形式。Cryo-EM 结构表明 CTPS2 细丝在活性和非活性形式之间动态切换,以响应底物和产物水平的变化。将细丝中许多 CTPS2 亚基的构象状态连接起来会导致高度协同调节,大大超过了单独的 CTPS2 四聚体的协同限制。这些结构揭示了构象和酶活性位点之间氨通道控制之间的联系,并解释人类 CTPS 亚型调控的差异。这种基于细丝的增强协同性机制证明了酶聚合的普遍现象如何能够适应不同的调节结果。
更新日期:2019-12-25
中文翻译:

耦合结构转变能够高度协同调节人类 CTPS2 细丝。
许多酶组装成特定的寡聚体,提供了一种协同调节活性的机制。最近的研究描述了一种调节模式,其中酶活性通过聚合成大规模细丝来调节。在这里,我们描述了人类 CTP 合酶 2 (CTPS2) 采用的一种基于聚合的超灵敏调节形式。Cryo-EM 结构表明 CTPS2 细丝在活性和非活性形式之间动态切换,以响应底物和产物水平的变化。将细丝中许多 CTPS2 亚基的构象状态连接起来会导致高度协同调节,大大超过了单独的 CTPS2 四聚体的协同限制。这些结构揭示了构象和酶活性位点之间氨通道控制之间的联系,并解释人类 CTPS 亚型调控的差异。这种基于细丝的增强协同性机制证明了酶聚合的普遍现象如何能够适应不同的调节结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号