当前位置:
X-MOL 学术
›
PLOS Genet.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A GWAS approach identifies Dapp1 as a determinant of air pollution-induced airway hyperreactivity.
PLOS Genetics ( IF 4.0 ) Pub Date : 2019-12-23 , DOI: 10.1371/journal.pgen.1008528 Hadi Maazi 1 , Jaana A Hartiala 2, 3 , Yuzo Suzuki 1 , Amanda L Crow 2, 3 , Pedram Shafiei Jahani 1 , Jonathan Lam 1 , Nisheel Patel 1 , Diamanda Rigas 1 , Yi Han 2, 3 , Pin Huang 2, 3 , Eleazar Eskin 4 , Aldons J Lusis 5, 6, 7 , Frank D Gilliland 2 , Omid Akbari 1 , Hooman Allayee 2, 3
PLOS Genetics ( IF 4.0 ) Pub Date : 2019-12-23 , DOI: 10.1371/journal.pgen.1008528 Hadi Maazi 1 , Jaana A Hartiala 2, 3 , Yuzo Suzuki 1 , Amanda L Crow 2, 3 , Pedram Shafiei Jahani 1 , Jonathan Lam 1 , Nisheel Patel 1 , Diamanda Rigas 1 , Yi Han 2, 3 , Pin Huang 2, 3 , Eleazar Eskin 4 , Aldons J Lusis 5, 6, 7 , Frank D Gilliland 2 , Omid Akbari 1 , Hooman Allayee 2, 3
Affiliation
|
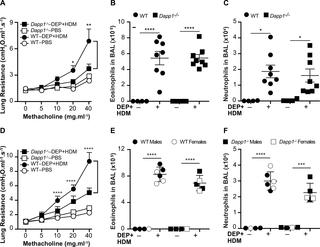
Asthma is a chronic inflammatory disease of the airways with contributions from genes, environmental exposures, and their interactions. While genome-wide association studies (GWAS) in humans have identified ~200 susceptibility loci, the genetic factors that modulate risk of asthma through gene-environment (GxE) interactions remain poorly understood. Using the Hybrid Mouse Diversity Panel (HMDP), we sought to identify the genetic determinants of airway hyperreactivity (AHR) in response to diesel exhaust particles (DEP), a model traffic-related air pollutant. As measured by invasive plethysmography, AHR under control and DEP-exposed conditions varied 3-4-fold in over 100 inbred strains from the HMDP. A GWAS with linear mixed models mapped two loci significantly associated with lung resistance under control exposure to chromosomes 2 (p = 3.0x10-6) and 19 (p = 5.6x10-7). The chromosome 19 locus harbors Il33 and is syntenic to asthma association signals observed at the IL33 locus in humans. A GxE GWAS for post-DEP exposure lung resistance identified a significantly associated locus on chromosome 3 (p = 2.5x10-6). Among the genes at this locus is Dapp1, an adaptor molecule expressed in immune-related and mucosal tissues, including the lung. Dapp1-deficient mice exhibited significantly lower AHR than control mice but only after DEP exposure, thus functionally validating Dapp1 as one of the genes underlying the GxE association at this locus. In summary, our results indicate that some of the genetic determinants for asthma-related phenotypes may be shared between mice and humans, as well as the existence of GxE interactions in mice that modulate lung function in response to air pollution exposures relevant to humans.
中文翻译:

GWAS方法将Dapp1识别为空气污染引起的气道高反应性的决定因素。
哮喘是一种慢性呼吸道炎性疾病,其发病原因包括基因,环境暴露及其相互作用。尽管人类的全基因组关联研究(GWAS)已确定约200个易感基因座,但通过基因环境(GxE)相互作用调节哮喘风险的遗传因素仍然知之甚少。我们使用混合小鼠多样性专家组(HMDP),试图确定响应于模型排气相关空气污染物柴油机尾气颗粒(DEP)的气道高反应性(AHR)的遗传决定因素。通过侵入性体积描记法测量,在HMDP的100多个自交系中,在对照和DEP暴露条件下,AHR发生了3到4倍的变化。具有线性混合模型的GWAS绘制了两个基因座,这些基因座在2号染色体的对照暴露下显着与肺耐药性相关(p = 3。0x10-6)和19(p = 5.6x10-7)。第19号染色体位点包含Il33,与在人类IL33基因座处观察到的哮喘关联信号同音。DEP暴露后肺耐药性的GxE GWAS确定了3号染色体上显着相关的基因座(p = 2.5x10-6)。在这个基因座的基因中有Dapp1,这是一种在免疫相关和粘膜组织(包括肺)中表达的衔接子分子。Dapp1缺陷小鼠表现出明显低于对照组的AHR,但仅在DEP暴露后才显示,因此在功能上验证Dapp1是该位点GxE关联的基础基因之一。总而言之,我们的结果表明,与哮喘有关的表型的某些遗传决定因素可能在小鼠和人类之间共享,
更新日期:2019-12-25
中文翻译:

GWAS方法将Dapp1识别为空气污染引起的气道高反应性的决定因素。
哮喘是一种慢性呼吸道炎性疾病,其发病原因包括基因,环境暴露及其相互作用。尽管人类的全基因组关联研究(GWAS)已确定约200个易感基因座,但通过基因环境(GxE)相互作用调节哮喘风险的遗传因素仍然知之甚少。我们使用混合小鼠多样性专家组(HMDP),试图确定响应于模型排气相关空气污染物柴油机尾气颗粒(DEP)的气道高反应性(AHR)的遗传决定因素。通过侵入性体积描记法测量,在HMDP的100多个自交系中,在对照和DEP暴露条件下,AHR发生了3到4倍的变化。具有线性混合模型的GWAS绘制了两个基因座,这些基因座在2号染色体的对照暴露下显着与肺耐药性相关(p = 3。0x10-6)和19(p = 5.6x10-7)。第19号染色体位点包含Il33,与在人类IL33基因座处观察到的哮喘关联信号同音。DEP暴露后肺耐药性的GxE GWAS确定了3号染色体上显着相关的基因座(p = 2.5x10-6)。在这个基因座的基因中有Dapp1,这是一种在免疫相关和粘膜组织(包括肺)中表达的衔接子分子。Dapp1缺陷小鼠表现出明显低于对照组的AHR,但仅在DEP暴露后才显示,因此在功能上验证Dapp1是该位点GxE关联的基础基因之一。总而言之,我们的结果表明,与哮喘有关的表型的某些遗传决定因素可能在小鼠和人类之间共享,











































 京公网安备 11010802027423号
京公网安备 11010802027423号