当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mild, Rapid, and Chemoselective Procedure for the Introduction of the 9-Phenyl-9-fluorenyl Protecting Group into Amines, Acids, Alcohols, Sulfonamides, Amides, and Thiols.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-12-24 , DOI: 10.1021/acs.joc.9b02809 Jacob Soley 1 , Scott D Taylor 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-12-24 , DOI: 10.1021/acs.joc.9b02809 Jacob Soley 1 , Scott D Taylor 1
Affiliation
|
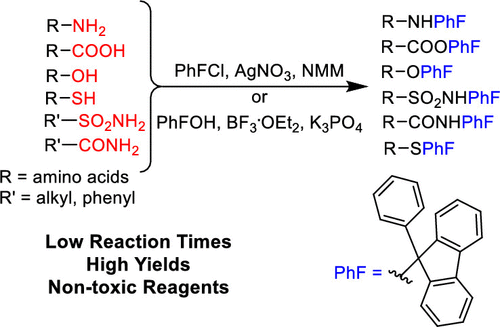
The 9-phenyl-9-fluorenyl (PhF) group has been used as an Nα protecting group of amino acids and their derivatives mainly as a result of its ability to prevent racemization. However, installing this group using the standard protocol, which employs 9-bromo-9-phenylfluorene/K3PO4/Pb(NO3)2, often takes days and yields can be variable. Here, we demonstrate that the PhF group can be introduced into the amino group of Weinreb's amides and methyl esters of amino acids, as well as into alcohols and carboxylic acids, rapidly and in excellent yields, using 9-chloro-9-phenylfluorene (PhFCl)/N-methylmorpholine (NMM)/AgNO3. Nα-PhF-protected amino acids can be prepared from unprotected α-amino acids, rapidly and often in near quantitative yields, by treatment with N,O-bis(trimethylsilyl)acetamide (BSA) and then PhFCl/NMM/AgNO3. Primary alcohols can be protected with the PhF group in the presence of secondary alcohols in moderate yield. Using PhFCl/AgNO3, a primary alcohol can be protected in good yield in the presence of a primary ammonium salt or a carboxylic acid. Primary sulfonamides and amides can be protected in moderate to good yields using phenylfluorenyl alcohol (PhFOH)/BF3·OEt2/K3PO4, while thiols can be protected in good to excellent yield using PhFOH/BF3·OEt2 even in the presence of a carboxylic acid or primary ammonium group.
中文翻译:

用于将9-苯基-9-芴基保护基引入胺,酸,醇,磺酰胺,酰胺和硫醇的温和,快速和化学选择性方法。
9-苯基-9-芴基(PhF)基团已被用作氨基酸及其衍生物的Nα保护基团,这主要是由于其具有防止外消旋作用的能力。但是,使用标准协议安装该组,该协议使用9-溴-9-苯基芴/ K3PO4 / Pb(NO3)2,通常需要几天的时间,并且产量可能会有所不同。在这里,我们证明了使用9-氯-9-苯基芴基(PhFCl )/ N-甲基吗啉(NMM)/ AgNO3。可以先用N,O-双(三甲基甲硅烷基)乙酰胺(BSA)然后用PhFCl / NMM / AgNO3处理,由未保护的α-氨基酸快速且经常以接近定量的收率制备Nα-PhF保护的氨基酸。在仲醇存在下,可以用PhF基团保护伯醇,且产率适中。使用PhFCl / AgNO3,可以在伯铵盐或羧酸的存在下以高收率保护伯醇。使用苯芴基醇(PhFOH)/ BF3·OEt2 / K3PO4可以以中等至良好的收率保护伯磺酰胺和酰胺,而使用PhFOH / BF3·OEt2则可以在良好的收率下保护巯基,即使在存在羧酸或初级铵基团。
更新日期:2020-01-14
中文翻译:

用于将9-苯基-9-芴基保护基引入胺,酸,醇,磺酰胺,酰胺和硫醇的温和,快速和化学选择性方法。
9-苯基-9-芴基(PhF)基团已被用作氨基酸及其衍生物的Nα保护基团,这主要是由于其具有防止外消旋作用的能力。但是,使用标准协议安装该组,该协议使用9-溴-9-苯基芴/ K3PO4 / Pb(NO3)2,通常需要几天的时间,并且产量可能会有所不同。在这里,我们证明了使用9-氯-9-苯基芴基(PhFCl )/ N-甲基吗啉(NMM)/ AgNO3。可以先用N,O-双(三甲基甲硅烷基)乙酰胺(BSA)然后用PhFCl / NMM / AgNO3处理,由未保护的α-氨基酸快速且经常以接近定量的收率制备Nα-PhF保护的氨基酸。在仲醇存在下,可以用PhF基团保护伯醇,且产率适中。使用PhFCl / AgNO3,可以在伯铵盐或羧酸的存在下以高收率保护伯醇。使用苯芴基醇(PhFOH)/ BF3·OEt2 / K3PO4可以以中等至良好的收率保护伯磺酰胺和酰胺,而使用PhFOH / BF3·OEt2则可以在良好的收率下保护巯基,即使在存在羧酸或初级铵基团。











































 京公网安备 11010802027423号
京公网安备 11010802027423号