当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phase Equilibrium of Carbon Dioxide Hydrates Inhibited with MEG and NaCl above the Upper Quadruple Point
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2019-12-24 , DOI: 10.1021/acs.jced.9b01001 Jose C. Cordeiro 1 , Moises A. Marcelino Neto 1 , Rigoberto E. M. Morales 1 , Amadeu K. Sum 2
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2019-12-24 , DOI: 10.1021/acs.jced.9b01001 Jose C. Cordeiro 1 , Moises A. Marcelino Neto 1 , Rigoberto E. M. Morales 1 , Amadeu K. Sum 2
Affiliation
|
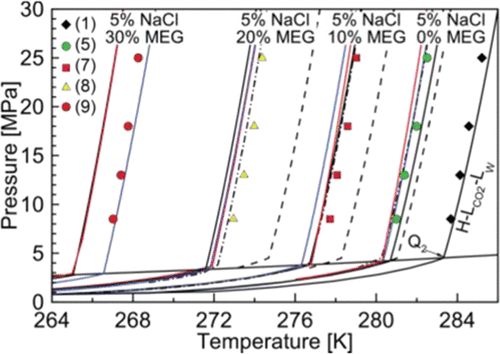
Exploration of oil fields with high concentrations of carbon dioxide brings new challenges to the oil and gas industry. The high pressures reached in production pipelines can cause the condensation of the gas phase, characterizing the appearance of an upper quadruple point in the hydrate phase diagram. To prevent the formation of hydrates, the use of thermodynamic inhibitors, such as alcohols and glycols, is common in the industry. In addition, water from oil production is naturally inhibited with salts, such as NaCl. In this work, equilibrium data not previously reported for CO2 hydrates inhibited with MEG and NaCl were determined for pressures between 8.5 and 25 MPa using an isobaric experimental procedure. By the Clausius–Clapeyron relation and concepts of water activity, the consistency of the data was evaluated with promising results. A comparison of the measured data obtained in this work with prediction tools (Multiflash, PVTsim, and CSMGem) and the model by Sirino et al.30 [ Fluid Phase Equilib. 2018, 475, 45−63] was done.
中文翻译:

MEG和NaCl抑制高四倍点以上的二氧化碳水合物的相平衡
高二氧化碳含量油田的勘探给石油和天然气工业带来了新的挑战。生产管道中达到的高压会引起气相冷凝,这在水合物相图中显示出较高的四倍点。为了防止水合物的形成,工业上通常使用热力学抑制剂,例如醇和二醇。此外,石油生产中的水自然会被盐(例如NaCl)抑制。在这项工作中,以前未报告过CO 2的平衡数据使用等压实验程序确定了MEG和NaCl抑制的水合物的压力在8.5和25 MPa之间。通过克劳修斯-克拉珀龙关系和水活度的概念,对数据的一致性进行了评估,并得出了令人满意的结果。使用预测工具(Multiflash,PVTsim和CSMGem)和Sirino等人的模型比较了这项工作中获得的测量数据。30 [液相平衡。 2018,475,45-63]已完成。
更新日期:2019-12-25
中文翻译:

MEG和NaCl抑制高四倍点以上的二氧化碳水合物的相平衡
高二氧化碳含量油田的勘探给石油和天然气工业带来了新的挑战。生产管道中达到的高压会引起气相冷凝,这在水合物相图中显示出较高的四倍点。为了防止水合物的形成,工业上通常使用热力学抑制剂,例如醇和二醇。此外,石油生产中的水自然会被盐(例如NaCl)抑制。在这项工作中,以前未报告过CO 2的平衡数据使用等压实验程序确定了MEG和NaCl抑制的水合物的压力在8.5和25 MPa之间。通过克劳修斯-克拉珀龙关系和水活度的概念,对数据的一致性进行了评估,并得出了令人满意的结果。使用预测工具(Multiflash,PVTsim和CSMGem)和Sirino等人的模型比较了这项工作中获得的测量数据。30 [液相平衡。 2018,475,45-63]已完成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号