当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water Networks and Correlated Motions in Mutant Isocitrate Dehydrogenase 1 (IDH1) Are Critical for Allosteric Inhibitor Binding and Activity.
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-13 , DOI: 10.1021/acs.biochem.9b01023 Jennifer M Chambers 1 , Wade Miller 2 , Giovanni Quichocho 3 , Viraj Upadhye 3 , Diego Avellaneda Matteo 3 , Andrey A Bobkov 4 , Christal D Sohl 3 , Jamie M Schiffer 5
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-13 , DOI: 10.1021/acs.biochem.9b01023 Jennifer M Chambers 1 , Wade Miller 2 , Giovanni Quichocho 3 , Viraj Upadhye 3 , Diego Avellaneda Matteo 3 , Andrey A Bobkov 4 , Christal D Sohl 3 , Jamie M Schiffer 5
Affiliation
|
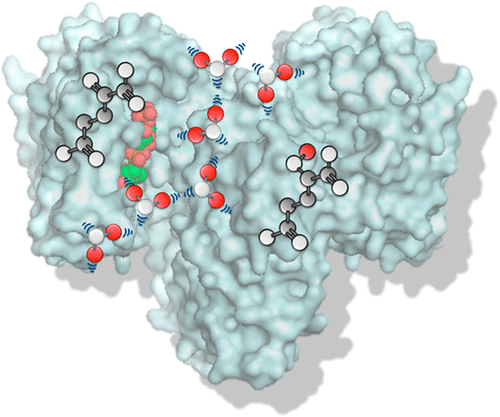
Point mutations in human isocitrate dehydrogenase 1 (IDH1) can drive malignancies, including lower-grade gliomas and secondary glioblastomas, chondrosarcomas, and acute myeloid leukemias. These mutations, which usually affect residue R132, ablate the normal activity of catalyzing the NADP+-dependent oxidation of isocitrate to α-ketoglutarate (αKG) while also acquiring a neomorphic activity of reducing αKG to d-2-hydroxyglutarate (D2HG). Mutant IDH1 can be selectively therapeutically targeted due to structural differences that occur in the wild type (WT) versus mutant form of the enzyme, though the full mechanisms of this selectivity are still under investigation. Here we probe the mechanistic features of the neomorphic activity and selective small molecule inhibition through a new lens, employing WaterMap and molecular dynamics simulations. These tools identified a high-energy path of water molecules connecting the inhibitor binding site with the αKG and NADP+ binding sites in mutant IDH1. This water path aligns spatially with the α10 helix from WT IDH1 crystal structures. Mutating residues at the termini of this water path specifically disrupted inhibitor binding and/or D2HG production, revealing additional key residues to consider in optimizing druglike molecules against mutant IDH1. Taken together, our findings from molecular simulations and mutant enzyme kinetic assays provide insight into how disrupting water paths through enzyme active sites can impact not only inhibitor potency but also substrate recognition and activity.
中文翻译:

突变异柠檬酸脱氢酶1(IDH1)中的水网络和相关运动对于变构抑制剂的结合和活性至关重要。
人异柠檬酸脱氢酶1(IDH1)的点突变可导致恶性肿瘤,包括低级神经胶质瘤和继发性胶质母细胞瘤,软骨肉瘤和急性髓细胞性白血病。这些通常影响残基R132的突变消除了催化NADP +依赖性异柠檬酸氧化为α-酮戊二酸(αKG)的正常活性,同时还获得了将αKG还原为d-2-羟基戊二酸(D2HG)的新形态活性。由于在野生型(WT)与突变形式的酶中存在结构差异,因此可以选择性地对突变IDH1进行治疗性靶向,尽管这种选择性的完整机制仍在研究中。在这里,我们使用WaterMap和分子动力学模拟,通过新的透镜探索新态活动和选择性小分子抑制的机制特征。这些工具确定了将抑制剂结合位点与突变体IDH1中的αKG和NADP +结合位点相连的水分子的高能路径。该水路与WT IDH1晶体结构的α10螺旋在空间上对齐。在该水通路末端的突变残基特异性破坏了抑制剂的结合和/或D2HG的产生,揭示了在优化针对突变IDH1的类药物分子时需要考虑的其他关键残基。两者合计,我们从分子模拟和突变酶动力学分析中获得的发现提供了洞悉通过酶活性位点的水路径如何不仅会影响抑制剂效力而且还会影响底物识别和活性的见解。该水路与WT IDH1晶体结构的α10螺旋在空间上对齐。在该水通路末端的突变残基特异性破坏了抑制剂的结合和/或D2HG的产生,揭示了在优化针对突变IDH1的类药物分子时需要考虑的其他关键残基。两者合计,我们从分子模拟和突变酶动力学分析中获得的发现提供了洞悉通过酶活性位点的水路径如何不仅会影响抑制剂效力而且还会影响底物识别和活性的见解。该水路与WT IDH1晶体结构的α10螺旋在空间上对齐。在该水通路末端的突变残基特异性破坏了抑制剂的结合和/或D2HG的产生,揭示了在优化针对突变IDH1的类药物分子时需要考虑的其他关键残基。两者合计,我们从分子模拟和突变酶动力学分析中获得的发现提供了洞悉通过酶活性位点的水路径如何不仅会影响抑制剂效力而且还会影响底物识别和活性的见解。
更新日期:2020-01-13
中文翻译:

突变异柠檬酸脱氢酶1(IDH1)中的水网络和相关运动对于变构抑制剂的结合和活性至关重要。
人异柠檬酸脱氢酶1(IDH1)的点突变可导致恶性肿瘤,包括低级神经胶质瘤和继发性胶质母细胞瘤,软骨肉瘤和急性髓细胞性白血病。这些通常影响残基R132的突变消除了催化NADP +依赖性异柠檬酸氧化为α-酮戊二酸(αKG)的正常活性,同时还获得了将αKG还原为d-2-羟基戊二酸(D2HG)的新形态活性。由于在野生型(WT)与突变形式的酶中存在结构差异,因此可以选择性地对突变IDH1进行治疗性靶向,尽管这种选择性的完整机制仍在研究中。在这里,我们使用WaterMap和分子动力学模拟,通过新的透镜探索新态活动和选择性小分子抑制的机制特征。这些工具确定了将抑制剂结合位点与突变体IDH1中的αKG和NADP +结合位点相连的水分子的高能路径。该水路与WT IDH1晶体结构的α10螺旋在空间上对齐。在该水通路末端的突变残基特异性破坏了抑制剂的结合和/或D2HG的产生,揭示了在优化针对突变IDH1的类药物分子时需要考虑的其他关键残基。两者合计,我们从分子模拟和突变酶动力学分析中获得的发现提供了洞悉通过酶活性位点的水路径如何不仅会影响抑制剂效力而且还会影响底物识别和活性的见解。该水路与WT IDH1晶体结构的α10螺旋在空间上对齐。在该水通路末端的突变残基特异性破坏了抑制剂的结合和/或D2HG的产生,揭示了在优化针对突变IDH1的类药物分子时需要考虑的其他关键残基。两者合计,我们从分子模拟和突变酶动力学分析中获得的发现提供了洞悉通过酶活性位点的水路径如何不仅会影响抑制剂效力而且还会影响底物识别和活性的见解。该水路与WT IDH1晶体结构的α10螺旋在空间上对齐。在该水通路末端的突变残基特异性破坏了抑制剂的结合和/或D2HG的产生,揭示了在优化针对突变IDH1的类药物分子时需要考虑的其他关键残基。两者合计,我们从分子模拟和突变酶动力学分析中获得的发现提供了洞悉通过酶活性位点的水路径如何不仅会影响抑制剂效力而且还会影响底物识别和活性的见解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号