当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Charge Movement and Structural Changes in the Gas-Phase Unfolding of Multimeric Protein Complexes Captured by Native Top-Down Mass Spectrometry.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2019-12-23 , DOI: 10.1021/acs.analchem.9b03469 Mowei Zhou 1 , Weijing Liu 1 , Jared B Shaw 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2019-12-23 , DOI: 10.1021/acs.analchem.9b03469 Mowei Zhou 1 , Weijing Liu 1 , Jared B Shaw 1
Affiliation
|
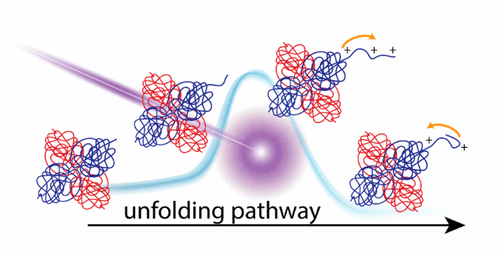
The extent to which noncovalent protein complexes retain native structure in the gas phase is highly dependent on experimental conditions. Energetic collisions with background gas can cause structural changes ranging from unfolding to subunit dissociation. Additionally, recent studies have highlighted the role of charge in such structural changes, but the mechanism is not completely understood. In this study, native top down (native TD) mass spectrometry was used to probe gas-phase structural changes of alcohol dehydrogenase (ADH, 4mer) under varying degrees of in-source activation. Changes in covalent backbone fragments produced by electron capture dissociation (ECD) or 193 nm ultraviolet photodissociation (UVPD) were attributed to structural changes of the ADH 4mer. ECD fragments indicated unfolding started at the N-terminus, and the charge states of UVPD fragments enabled monitoring of charge migration to the unfolded regions. Interestingly, UVPD fragments also indicated that the charge at the "unfolding" N-terminus of ADH decreased at high in-source activation energies after the initial increase. We proposed a possible "refolding-after-unfolding" mechanism, as further supported by monitoring hydrogen elimination from radical a-ions produced by UVPD at the N-terminus of ADH. However, "refolding-after-unfolding" with increasing in-source activation was not observed for charge-reduced ADH, which likely adopted compact structures that are resistant to both charge migration and unfolding. When combined, these results support a charge-directed unfolding mechanism for protein complexes. Overall, an experimental framework was outlined for utilizing native TD to generate structure-informative mass spectral signatures for protein complexes that complement other structure characterization techniques, such as ion mobility and computational modeling.
中文翻译:

通过自然自上而下的质谱技术捕获的多聚蛋白质复合物的气相展开中的电荷运动和结构变化。
非共价蛋白复合物在气相中保留天然结构的程度在很大程度上取决于实验条件。与背景气体的强烈碰撞会导致结构变化,从展开到亚基解离。另外,最近的研究强调了电荷在这种结构变化中的作用,但其机理尚不完全清楚。在这项研究中,使用自然自上而下(天然TD)质谱法来探测在不同程度的源内活化作用下酒精脱氢酶(ADH,4mer)的气相结构变化。电子捕获解离(ECD)或193 nm紫外光解离(UVPD)产生的共价主链片段的变化归因于ADH 4mer的结构变化。ECD片段指示在N端开始展开,UVPD碎片的电荷状态可以监测电荷向未折叠区域的迁移。有趣的是,UVPD片段还表明,在初始增加后,ADH在“未折叠的” N端的电荷在高源内活化能下降低了。我们提出了一种可能的“展开后折叠”机制,进一步监测了在ADH的N端从UVPD产生的自由基a离子中氢的消除。然而,对于电荷减少的ADH,未观察到随着源内活化增加而发生的“折叠后折叠”,其可能采用了紧凑的结构,既能抵抗电荷迁移又能抵抗折叠。当结合在一起时,这些结果支持蛋白质复合物的电荷导向的展开机制。全面的,
更新日期:2020-01-08
中文翻译:

通过自然自上而下的质谱技术捕获的多聚蛋白质复合物的气相展开中的电荷运动和结构变化。
非共价蛋白复合物在气相中保留天然结构的程度在很大程度上取决于实验条件。与背景气体的强烈碰撞会导致结构变化,从展开到亚基解离。另外,最近的研究强调了电荷在这种结构变化中的作用,但其机理尚不完全清楚。在这项研究中,使用自然自上而下(天然TD)质谱法来探测在不同程度的源内活化作用下酒精脱氢酶(ADH,4mer)的气相结构变化。电子捕获解离(ECD)或193 nm紫外光解离(UVPD)产生的共价主链片段的变化归因于ADH 4mer的结构变化。ECD片段指示在N端开始展开,UVPD碎片的电荷状态可以监测电荷向未折叠区域的迁移。有趣的是,UVPD片段还表明,在初始增加后,ADH在“未折叠的” N端的电荷在高源内活化能下降低了。我们提出了一种可能的“展开后折叠”机制,进一步监测了在ADH的N端从UVPD产生的自由基a离子中氢的消除。然而,对于电荷减少的ADH,未观察到随着源内活化增加而发生的“折叠后折叠”,其可能采用了紧凑的结构,既能抵抗电荷迁移又能抵抗折叠。当结合在一起时,这些结果支持蛋白质复合物的电荷导向的展开机制。全面的,











































 京公网安备 11010802027423号
京公网安备 11010802027423号