当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anodic Oxidation for the Stereoselective Synthesis of Heterocycles.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-12-24 , DOI: 10.1021/acs.accounts.9b00513 Kosuke Yamamoto 1 , Masami Kuriyama 1 , Osamu Onomura 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-12-24 , DOI: 10.1021/acs.accounts.9b00513 Kosuke Yamamoto 1 , Masami Kuriyama 1 , Osamu Onomura 1
Affiliation
|
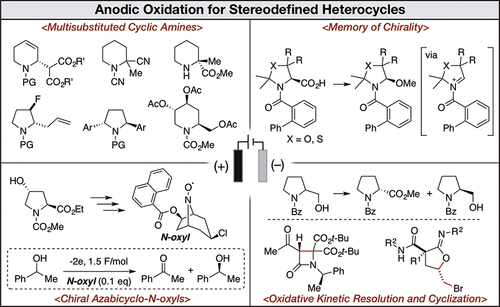
Stereodefined aliphatic heterocycles are one of the fundamental structural motifs observed in natural products and biologically active compounds. Various strategies for the synthesis of these building blocks based on transition metal catalysis, organocatalysis, and noncatalytic conditions have been developed. Although electrosynthesis has also been utilized for the functionalization of aliphatic heterocycles, stereoselective transformations under electrochemical conditions are still a challenging field in electroorganic chemistry. This Account consists of four main topics related to our recent efforts on the diastereo- and/or enantioselective synthesis of aliphatic heterocycles, especially N-heterocycles, using anodic oxidations as key steps. The first topic is the development of stereoselective synthetic methods for multisubstituted piperidines and pyrrolidines from anodically prepared α-methoxy cyclic amines. Our strategies were based primarily on N-acyliminium ion chemistry, and the key electrochemical transformations were diastereoselective anodic methoxylation, diastereoselective arylation, and anodic deallylative methoxylation. Furthermore, we found a unique property of the N-cyano protecting group that enabled the electrochemical α-methoxylation of α-substituted cyclic amines. The second topic of investigation is memory of chirality in electrochemical decarboxylative methoxylation. We observed that the electrochemical decarboxylative methoxylation of oxazolidine and thiazolidine derivatives with the appropriate N-protecting group occurred in a stereospecific manner even though the reaction proceeded through an sp2 planar carbon center. Our findings demonstrated the first example of memory of chirality in N-acyliminium ion chemistry. The third topic is the synthesis of chiral azabicyclo-N-oxyls and their application to chiral organocatalysis in the electrochemical oxidative kinetic resolution of secondary alcohols. The final topic is stereoselective transformations utilizing anodically generated halogen cations. We investigated the oxidative kinetic resolution of amino alcohol derivatives using anodically generated bromo cations. We also developed an intramolecular C-C bond formation of keto amides, a diastereoselective bromoiminolactonization of α-allyl malonamides, and an oxidative ring expansion reaction of allyl alcohols. It is noteworthy that most of the electrochemical reactions were performed in undivided cells under constant-current conditions, which avoided a complicated reaction setup and was beneficial for a large-scale reaction. In addition, we developed some enantioselective electrochemical transformations that are still challenges in electroorganic chemistry. We hope that our research will contribute to the further development of diastereo- and/or enantioselective transformations and the construction of valuable heterocyclic compounds using an electrochemical approach.
中文翻译:

杂环立体合成的阳极氧化。
立体定义的脂族杂环是在天然产物和生物活性化合物中观察到的基本结构基序之一。已经开发了基于过渡金属催化,有机催化和非催化条件合成这些构件的各种策略。尽管电合成也已经用于脂肪族杂环的功能化,但是在电化学条件下的立体选择性转化仍然是有机化学中一个具有挑战性的领域。该帐户包括四个主要主题,这些主题与我们最近使用阳极氧化作为关键步骤的脂族杂环(尤其是N-杂环)的非对映和/或对映选择性合成有关。第一个主题是开发由阳极制备的α-甲氧基环胺用于多取代哌啶和吡咯烷的立体选择性合成方法。我们的策略主要基于N-酰基亚胺离子化学,关键的电化学转变是非对映选择性阳极甲氧基化,非对映选择性芳基化和阳极脱羧甲氧基化。此外,我们发现了N-氰基保护基的独特性质,该性质使α-取代的环胺能够进行电化学α-甲氧基化。研究的第二个主题是电化学脱羧甲氧基化中的手性记忆。我们观察到,即使反应通过sp2平面碳中心进行,恶唑烷和噻唑烷衍生物具有适当的N-保护基的电化学脱羧甲氧基化也以立体有择方式发生。我们的发现证明了N-酰基酰亚胺离子化学中手性记忆的第一个例子。第三个主题是手性氮杂双环-N-氧基的合成及其在仲醇电化学氧化动力学拆分中的手性有机催化应用。最后一个主题是利用阳极产生的卤素阳离子进行的立体选择性转化。我们研究了使用阳极产生的溴阳离子对氨基醇衍生物的氧化动力学拆分。我们还开发了酮酰胺的分子内CC键形成,α-烯丙基丙二酰胺的非对映选择性溴亚氨基内酯化,以及烯丙醇的氧化扩环反应。值得注意的是,大多数电化学反应是在恒定电流条件下在未分裂的电池中进行的,这避免了复杂的反应设置,并且有利于大规模反应。另外,我们开发了一些对映选择性的电化学转化,这些转化仍然是有机化学中的挑战。我们希望我们的研究将有助于非对映和/或对映选择性转化的进一步发展,以及使用电化学方法构建有价值的杂环化合物。值得注意的是,大多数电化学反应是在恒定电流条件下在未分裂的电池中进行的,这避免了复杂的反应设置,并且有利于大规模反应。另外,我们开发了一些对映选择性的电化学转化,这些转化仍然是有机化学中的挑战。我们希望我们的研究将有助于非对映和/或对映选择性转化的进一步发展,以及使用电化学方法构建有价值的杂环化合物。值得注意的是,大多数电化学反应是在恒定电流条件下在未分裂的电池中进行的,这避免了复杂的反应设置,并且有利于大规模反应。另外,我们开发了一些对映选择性的电化学转化,这些转化仍然是有机化学中的挑战。我们希望我们的研究将有助于非对映和/或对映选择性转化的进一步发展,以及使用电化学方法构建有价值的杂环化合物。我们开发了一些对映选择性的电化学转变,这些转变仍然是有机化学中的挑战。我们希望我们的研究将有助于非对映和/或对映选择性转化的进一步发展,以及使用电化学方法构建有价值的杂环化合物。我们开发了一些对映选择性的电化学转变,这些转变仍然是有机化学中的挑战。我们希望我们的研究将有助于非对映和/或对映选择性转化的进一步发展,以及使用电化学方法构建有价值的杂环化合物。
更新日期:2019-12-25
中文翻译:

杂环立体合成的阳极氧化。
立体定义的脂族杂环是在天然产物和生物活性化合物中观察到的基本结构基序之一。已经开发了基于过渡金属催化,有机催化和非催化条件合成这些构件的各种策略。尽管电合成也已经用于脂肪族杂环的功能化,但是在电化学条件下的立体选择性转化仍然是有机化学中一个具有挑战性的领域。该帐户包括四个主要主题,这些主题与我们最近使用阳极氧化作为关键步骤的脂族杂环(尤其是N-杂环)的非对映和/或对映选择性合成有关。第一个主题是开发由阳极制备的α-甲氧基环胺用于多取代哌啶和吡咯烷的立体选择性合成方法。我们的策略主要基于N-酰基亚胺离子化学,关键的电化学转变是非对映选择性阳极甲氧基化,非对映选择性芳基化和阳极脱羧甲氧基化。此外,我们发现了N-氰基保护基的独特性质,该性质使α-取代的环胺能够进行电化学α-甲氧基化。研究的第二个主题是电化学脱羧甲氧基化中的手性记忆。我们观察到,即使反应通过sp2平面碳中心进行,恶唑烷和噻唑烷衍生物具有适当的N-保护基的电化学脱羧甲氧基化也以立体有择方式发生。我们的发现证明了N-酰基酰亚胺离子化学中手性记忆的第一个例子。第三个主题是手性氮杂双环-N-氧基的合成及其在仲醇电化学氧化动力学拆分中的手性有机催化应用。最后一个主题是利用阳极产生的卤素阳离子进行的立体选择性转化。我们研究了使用阳极产生的溴阳离子对氨基醇衍生物的氧化动力学拆分。我们还开发了酮酰胺的分子内CC键形成,α-烯丙基丙二酰胺的非对映选择性溴亚氨基内酯化,以及烯丙醇的氧化扩环反应。值得注意的是,大多数电化学反应是在恒定电流条件下在未分裂的电池中进行的,这避免了复杂的反应设置,并且有利于大规模反应。另外,我们开发了一些对映选择性的电化学转化,这些转化仍然是有机化学中的挑战。我们希望我们的研究将有助于非对映和/或对映选择性转化的进一步发展,以及使用电化学方法构建有价值的杂环化合物。值得注意的是,大多数电化学反应是在恒定电流条件下在未分裂的电池中进行的,这避免了复杂的反应设置,并且有利于大规模反应。另外,我们开发了一些对映选择性的电化学转化,这些转化仍然是有机化学中的挑战。我们希望我们的研究将有助于非对映和/或对映选择性转化的进一步发展,以及使用电化学方法构建有价值的杂环化合物。值得注意的是,大多数电化学反应是在恒定电流条件下在未分裂的电池中进行的,这避免了复杂的反应设置,并且有利于大规模反应。另外,我们开发了一些对映选择性的电化学转化,这些转化仍然是有机化学中的挑战。我们希望我们的研究将有助于非对映和/或对映选择性转化的进一步发展,以及使用电化学方法构建有价值的杂环化合物。我们开发了一些对映选择性的电化学转变,这些转变仍然是有机化学中的挑战。我们希望我们的研究将有助于非对映和/或对映选择性转化的进一步发展,以及使用电化学方法构建有价值的杂环化合物。我们开发了一些对映选择性的电化学转变,这些转变仍然是有机化学中的挑战。我们希望我们的研究将有助于非对映和/或对映选择性转化的进一步发展,以及使用电化学方法构建有价值的杂环化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号