当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activity-Based Sensing for Site-Specific Proteomic Analysis of Cysteine Oxidation.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-12-23 , DOI: 10.1021/acs.accounts.9b00562 Yunlong Shi 1 , Kate S Carroll 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-12-23 , DOI: 10.1021/acs.accounts.9b00562 Yunlong Shi 1 , Kate S Carroll 1
Affiliation
|
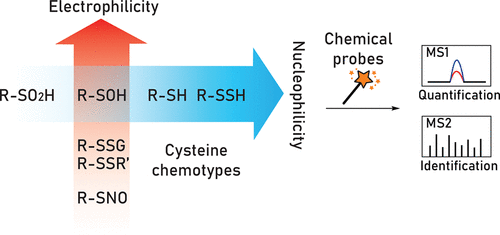
Oxidative post-translational modifications (OxiPTMs) of cysteine residues are the molecular foundation of thiol-based redox regulation that modulates physiological events such as cell proliferation, differentiation, and migration and, when dysregulated, can lead to biomolecule damage and cell death. Common OxiPTMs of cysteine thiols (-SH) include reversible modifications such as S-sulfenylation (-SOH), S-glutathionylation (-SSG), disulfide formation (-SSR), S-nitrosylation (-SNO), and S-sulfhydration (-SSH) as well as more biologically stable modifications like S-sulfinylation (-SO2H) and S-sulfonylation (-SO3H). In the past decade, our laboratory has developed first-in-class chemistry-based tools and proteomic methods to advance the field of thiol-based redox biology and oxidative stress. In this Account, we take the reader through the historical aspects of probe development and application in our laboratory, highlighting key advances in our understanding of sulfur chemistry, in the test tube and in living systems. Offering superior resolution, throughput, accuracy, and reproducibility, mass spectrometry (MS)-based proteomics coupled to chemoselective "activity-based" small-molecule probes is the most rigorous technique for global mapping of cysteine OxiPTMs. Herein, we describe the evolution of this field from indirect detection to state-of-the-art site-centric quantitative chemoproteomic approaches that enable mapping of physiological and pathological changes in cysteine oxidation. These methods enable protein and site-level identification, mechanistic studies, mapping fold-changes, and modification stoichiometry. In particular, this Account focuses on activity-based methods for profiling S-sulfenylation, S-sulfinylation, and S-sulfhydration with an eye toward new reactions and methodologies developed in our group as well as their applications that have shed new light on fundamental processes of redox biology. Among several classes of sulfenic acid probes, dimedone-based C-nucleophiles possess superior chemical selectivity and compatibility with tandem MS. Cell-permeable dimedone derivatives with a bioconjugation handle are capable of detecting of S-sulfenylation in living cells. In-depth screening of a C-nucleophile library has yielded several entities with significantly enhanced reactivity over dimedone while maintaining selectivity, and reversible linear C-nucleophiles that enable controlled target release. C-Nucleophiles have also been implemented in tag-switch methods to detect S-sulfhydration. Most recently, activity-based detection of protein S-sulfinylation with electrophilic nitrogen species (ENS), such as C-nitroso compounds and electron deficient diazines, offers significant advantages in simplicity-of-use and target specificity compared to label-free methods. When feasible, the rich information provided by site-centric quantitative proteomics should not be tainted by oxidation artifacts from cell lysis. Therefore, chemoselective probes that function in a native environment with low cytotoxicity, good cell-permeability, and competitive kinetics are desired in modern redox chemoproteomics approaches. As our understanding of sulfur chemistry and redox signaling evolves, newly discovered cysteine OxiPTMs in microorganisms, plants, cells, tissues, and disease models should innovatively promote mechanistic and therapeutic research.
中文翻译:

半胱氨酸氧化的基于位置的蛋白质组学分析的基于活动的传感。
半胱氨酸残基的氧化后翻译修饰(OxiPTM)是基于硫醇的氧化还原调节的分子基础,其调节生理事件,例如细胞增殖,分化和迁移,并且如果失调,可能导致生物分子损伤和细胞死亡。半胱氨酸硫醇(-SH)的常见OxiPTM包括可逆修饰,例如S-亚磺酰基化(-SOH),S-谷胱甘肽化(-SSG),二硫键形成(-SSR),S-亚硝基化(-SNO)和S-巯基化( -SSH)以及更具生物学稳定性的修饰,例如S-亚磺酰基化(-SO2H)和S-磺酰基化(-SO3H)。在过去的十年中,我们的实验室开发了一流的基于化学的工具和蛋白质组学方法,以推动基于硫醇的氧化还原生物学和氧化应激领域的发展。在这个帐户中,我们将引导读者了解实验室中探针开发和应用的历史方面,重点介绍我们对硫化学,试管和生物系统的理解方面的关键进展。基于质谱(MS)的蛋白质组学与化学选择性的“基于活性”的小分子探针相结合,可提供卓越的分辨率,通量,准确性和可重复性,是对半胱氨酸OxiPTM进行全局作图的最严格技术。本文中,我们描述了该领域从间接检测到以站点为中心的定量化学旋转方法的发展,该方法可以绘制半胱氨酸氧化的生理和病理变化。这些方法使蛋白质和位点水平鉴定,机理研究,作图倍数变化和修饰化学计量成为可能。特别是,本帐户重点介绍基于活动的S-亚磺酰化,S-亚磺酰化和S-巯基化分析方法,着眼于我们小组开发的新反应和新方法,以及它们的应用为氧化还原生物学的基本过程提供了新的思路。在几类亚硫酸探针中,基于二甲酮的C-亲核试剂具有出色的化学选择性和与串联MS的相容性。具有生物缀合手柄的可渗透细胞的二甲酮衍生物能够检测活细胞中的S-亚磺酰基化。深入筛选C-亲核分子文库已得到几个实体,其活性比二甲酮显着提高,同时保持了选择性,并且具有可逆的线性C-亲核体,能够控制靶标释放。C-Nucleophiles也已在标签转换方法中实现,以检测S-巯基化。最近,与无标记方法相比,使用亲电氮物种(ENS)(例如C-亚硝基化合物和电子缺乏的二嗪)对蛋白质S-亚磺酰化进行基于活性的检测,在使用简单性和靶标特异性方面具有显着优势。在可行的情况下,以位点为中心的蛋白质组学提供的丰富信息不应被细胞裂解产生的氧化伪影所污染。因此,在现代氧化还原化学蛋白质组学方法中需要在低细胞毒性,良好的细胞渗透性和竞争动力学的天然环境中起作用的化学选择性探针。随着我们对硫化学和氧化还原信号的理解的发展,微生物,植物,细胞,
更新日期:2019-12-25
中文翻译:

半胱氨酸氧化的基于位置的蛋白质组学分析的基于活动的传感。
半胱氨酸残基的氧化后翻译修饰(OxiPTM)是基于硫醇的氧化还原调节的分子基础,其调节生理事件,例如细胞增殖,分化和迁移,并且如果失调,可能导致生物分子损伤和细胞死亡。半胱氨酸硫醇(-SH)的常见OxiPTM包括可逆修饰,例如S-亚磺酰基化(-SOH),S-谷胱甘肽化(-SSG),二硫键形成(-SSR),S-亚硝基化(-SNO)和S-巯基化( -SSH)以及更具生物学稳定性的修饰,例如S-亚磺酰基化(-SO2H)和S-磺酰基化(-SO3H)。在过去的十年中,我们的实验室开发了一流的基于化学的工具和蛋白质组学方法,以推动基于硫醇的氧化还原生物学和氧化应激领域的发展。在这个帐户中,我们将引导读者了解实验室中探针开发和应用的历史方面,重点介绍我们对硫化学,试管和生物系统的理解方面的关键进展。基于质谱(MS)的蛋白质组学与化学选择性的“基于活性”的小分子探针相结合,可提供卓越的分辨率,通量,准确性和可重复性,是对半胱氨酸OxiPTM进行全局作图的最严格技术。本文中,我们描述了该领域从间接检测到以站点为中心的定量化学旋转方法的发展,该方法可以绘制半胱氨酸氧化的生理和病理变化。这些方法使蛋白质和位点水平鉴定,机理研究,作图倍数变化和修饰化学计量成为可能。特别是,本帐户重点介绍基于活动的S-亚磺酰化,S-亚磺酰化和S-巯基化分析方法,着眼于我们小组开发的新反应和新方法,以及它们的应用为氧化还原生物学的基本过程提供了新的思路。在几类亚硫酸探针中,基于二甲酮的C-亲核试剂具有出色的化学选择性和与串联MS的相容性。具有生物缀合手柄的可渗透细胞的二甲酮衍生物能够检测活细胞中的S-亚磺酰基化。深入筛选C-亲核分子文库已得到几个实体,其活性比二甲酮显着提高,同时保持了选择性,并且具有可逆的线性C-亲核体,能够控制靶标释放。C-Nucleophiles也已在标签转换方法中实现,以检测S-巯基化。最近,与无标记方法相比,使用亲电氮物种(ENS)(例如C-亚硝基化合物和电子缺乏的二嗪)对蛋白质S-亚磺酰化进行基于活性的检测,在使用简单性和靶标特异性方面具有显着优势。在可行的情况下,以位点为中心的蛋白质组学提供的丰富信息不应被细胞裂解产生的氧化伪影所污染。因此,在现代氧化还原化学蛋白质组学方法中需要在低细胞毒性,良好的细胞渗透性和竞争动力学的天然环境中起作用的化学选择性探针。随着我们对硫化学和氧化还原信号的理解的发展,微生物,植物,细胞,











































 京公网安备 11010802027423号
京公网安备 11010802027423号