当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Origin of Maleic Anhydride Selectivity during the Oxidative Scission of Levulinic Acid
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-08 , DOI: 10.1021/acscatal.9b04289 Ran Zhu 1 , Anargyros Chatzidimitriou 1 , Bowei Liu 1 , Deborah J. Kerwood 2 , Jesse Q. Bond 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-08 , DOI: 10.1021/acscatal.9b04289 Ran Zhu 1 , Anargyros Chatzidimitriou 1 , Bowei Liu 1 , Deborah J. Kerwood 2 , Jesse Q. Bond 1
Affiliation
|
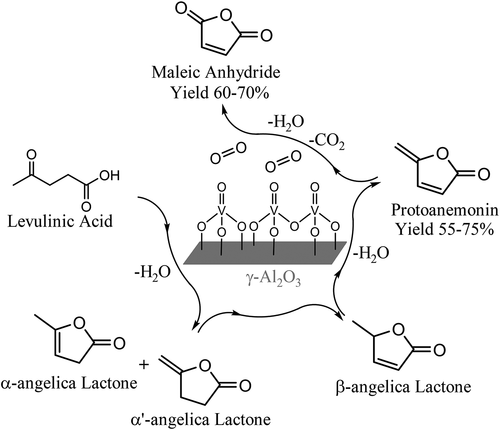
Biomass-derived levulinic acid (LA) is a green platform chemical, and we have previously reported an oxidative scission pathway that selectively transforms it into maleic anhydride (MA). This reaction is curious because it requires oxidative scission of the terminal (methyl) carbon in levulinic acid, whereas gas-phase methyl ketone oxidations are typically selective toward internal (alkyl) bond scission. In order to probe the origin of this disparity, we consider trends observed during the oxidative scission of ketones, keto acids, and keto acid analogues, and we highlight influences of steric hindrances, α-carbon substitution, and the presence of a secondary carboxylic acid functionality. We further consider the role of cyclic intermediates, namely Angelica lactones, in mediating selectivity during the oxidative scission of levulinic acid. Our kinetic analysis is supported by FTIR spectroscopy, which reveals the formation of hydrogen-deficient surface intermediates prior to the onset of oxidative scission. Finally, we pair short-contact-time selectivity analysis with GCMS and NMR spectroscopy to identify a previously undisclosed reaction intermediate—protoanemonin—that forms during the oxidative scission of levulinic acid and α-Angelica lactone. We conclude that facile oxidative dehydrogenation of β-Angelica lactone to form protoanemonin is the major driving force for the high selectivity toward methyl scission during levulinic acid oxidation. We also note that protoanemonin is an intriguing polyfunctional molecule that appears well-suited to bio-based production, and we have observed that it can be synthesized in yields from 55% to 75% (albeit at low concentration presently) during periods of transient reactor operation.
中文翻译:

理解乙酰丙酸氧化分裂过程中马来酸酐选择性的起源
生物质乙酰丙酸(LA)是一种绿色平台化学品,我们之前已经报道了一种氧化分裂途径,该途径有选择地将其转化为马来酸酐(MA)。该反应是令人好奇的,因为它需要在乙酰丙酸中氧化末端(甲基)碳,而气相的甲基酮氧化通常对内部(烷基)键具有选择性。为了探究这种差异的根源,我们考虑了在酮,酮酸和酮酸类似物的氧化断裂过程中观察到的趋势,并重点介绍了位阻,α-碳取代和仲羧酸的存在的影响。功能。我们进一步考虑了环状中间体即当归内酯在乙酰丙酸氧化断裂过程中介导选择性中的作用。我们的动力学分析得到了FTIR光谱学的支持,FTIR光谱学揭示了在发生氧化分裂之前,缺乏氢的表面中间体的形成。最后,我们将短接触时间选择性分析与GCMS和NMR光谱结合使用,以鉴定以前未公开的反应中间体原蛋黄素,该中间体在乙酰丙酸和α-当归内酯的氧化分裂过程中形成。我们得出结论,β-当归内酯的容易的氧化脱氢以形成原褐烟碱是乙酰丙酸氧化过程中对甲基断裂的高选择性的主要驱动力。我们还注意到,Protoanemonin是一种引人入胜的多功能分子,看起来非常适合生物基生产,
更新日期:2020-01-08
中文翻译:

理解乙酰丙酸氧化分裂过程中马来酸酐选择性的起源
生物质乙酰丙酸(LA)是一种绿色平台化学品,我们之前已经报道了一种氧化分裂途径,该途径有选择地将其转化为马来酸酐(MA)。该反应是令人好奇的,因为它需要在乙酰丙酸中氧化末端(甲基)碳,而气相的甲基酮氧化通常对内部(烷基)键具有选择性。为了探究这种差异的根源,我们考虑了在酮,酮酸和酮酸类似物的氧化断裂过程中观察到的趋势,并重点介绍了位阻,α-碳取代和仲羧酸的存在的影响。功能。我们进一步考虑了环状中间体即当归内酯在乙酰丙酸氧化断裂过程中介导选择性中的作用。我们的动力学分析得到了FTIR光谱学的支持,FTIR光谱学揭示了在发生氧化分裂之前,缺乏氢的表面中间体的形成。最后,我们将短接触时间选择性分析与GCMS和NMR光谱结合使用,以鉴定以前未公开的反应中间体原蛋黄素,该中间体在乙酰丙酸和α-当归内酯的氧化分裂过程中形成。我们得出结论,β-当归内酯的容易的氧化脱氢以形成原褐烟碱是乙酰丙酸氧化过程中对甲基断裂的高选择性的主要驱动力。我们还注意到,Protoanemonin是一种引人入胜的多功能分子,看起来非常适合生物基生产,











































 京公网安备 11010802027423号
京公网安备 11010802027423号