当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Dihydropinidine Enabled by Concurrent Multienzyme Catalysis and a Biocatalytic Alternative to Krapcho Dealkoxycarbonylation
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-08 , DOI: 10.1021/acscatal.9b04611 Natália Alvarenga 1, 2 , Stefan E. Payer 2 , Philipp Petermeier 2 , Christoph Kohlfuerst 2 , André Luiz Meleiro Porto 1 , Joerg H. Schrittwieser 2 , Wolfgang Kroutil 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-01-08 , DOI: 10.1021/acscatal.9b04611 Natália Alvarenga 1, 2 , Stefan E. Payer 2 , Philipp Petermeier 2 , Christoph Kohlfuerst 2 , André Luiz Meleiro Porto 1 , Joerg H. Schrittwieser 2 , Wolfgang Kroutil 2
Affiliation
|
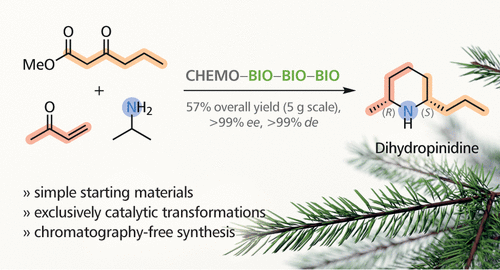
Dihydropinidine is a piperidine alkaloid found in spruce needles that has shown promising antifeedant activity against the large pine weevil, a widespread and economically relevant pest of coniferous tree plantations. Chemo-enzymatic approaches have previously been shown to enable a step-economic access to both enantiomers of this alkaloid, but the scalability of these syntheses is limited. Herein, we report a chemo-enzymatic route to dihydropinidine that is dominated by biocatalytic steps and affords the target alkaloid in excellent stereoisomeric purity (>99% ee and de) and high yield (57% overall) on multigram scale. Our synthesis makes use of a solvent-free, Lewis acid-catalyzed Michael addition and a biocatalytic alternative to Krapcho dealkoxycarbonylation, achieved by pig liver esterase (PLE)-catalyzed ester hydrolysis and acidification, and specifically developed for this purpose, to access a key intermediate, nonane-2,6-dione. This diketone is then converted into dihydropinidine by a concurrent one-pot (cascade) biotransformation catalyzed by a transaminase, an imine reductase, and an alcohol dehydrogenase. High yields and excellent regio- and stereoselectivities of the individual transformations render chromatographic purification of intermediates unnecessary and make it possible to carry out the entire sequence with a final hydrochloride precipitation of the target alkaloid as the sole purification step.
中文翻译:

并发多酶催化实现二氢吡啶的不对称合成和Krapcho脱烷氧基羰基化的生物催化替代方法。
二氢吡啶是一种在云杉针叶中发现的哌啶生物碱,对大松象鼻是一种有希望的拒食活性,大松象鼻是一种针叶树人工林的广泛且与经济相关的害虫。先前已显示化学酶法可以经济地逐步获得该生物碱的两种对映体,但这些合成方法的可扩展性受到限制。在本文中,我们报告了以化学催化途径主导的二氢吡啶的化学酶促路线,该路线以生物催化步骤为主,并以优良的立体异构纯度(> 99%ee和de)和高收率(总体为57%)提供目标生物碱(以克计)。我们的合成方法采用无溶剂,路易斯酸催化的迈克尔加成反应,以及通过猪肝酯酶(PLE)催化的酯水解和酸化作用实现的Krapcho脱氧羰基化的生物催化替代物,专门为此目的而开发的,用于访问关键中间体壬烷2,6-二酮。然后通过转氨酶,亚胺还原酶和醇脱氢酶催化的同时一锅(级联)生物转化法将该二酮转化为二氢吡啶。单个转化的高收率和出色的区域选择性和立体选择性使中间体的色谱纯化成为不必要,并且有可能以目标生物碱的最终盐酸盐沉淀作为唯一的纯化步骤来进行整个序列。
更新日期:2020-01-08
中文翻译:

并发多酶催化实现二氢吡啶的不对称合成和Krapcho脱烷氧基羰基化的生物催化替代方法。
二氢吡啶是一种在云杉针叶中发现的哌啶生物碱,对大松象鼻是一种有希望的拒食活性,大松象鼻是一种针叶树人工林的广泛且与经济相关的害虫。先前已显示化学酶法可以经济地逐步获得该生物碱的两种对映体,但这些合成方法的可扩展性受到限制。在本文中,我们报告了以化学催化途径主导的二氢吡啶的化学酶促路线,该路线以生物催化步骤为主,并以优良的立体异构纯度(> 99%ee和de)和高收率(总体为57%)提供目标生物碱(以克计)。我们的合成方法采用无溶剂,路易斯酸催化的迈克尔加成反应,以及通过猪肝酯酶(PLE)催化的酯水解和酸化作用实现的Krapcho脱氧羰基化的生物催化替代物,专门为此目的而开发的,用于访问关键中间体壬烷2,6-二酮。然后通过转氨酶,亚胺还原酶和醇脱氢酶催化的同时一锅(级联)生物转化法将该二酮转化为二氢吡啶。单个转化的高收率和出色的区域选择性和立体选择性使中间体的色谱纯化成为不必要,并且有可能以目标生物碱的最终盐酸盐沉淀作为唯一的纯化步骤来进行整个序列。











































 京公网安备 11010802027423号
京公网安备 11010802027423号