当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site-Specific Siderocalin Binding to Ferric and Ferric-Free Enterobactin As Revealed by Mass Spectrometry.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-01-13 , DOI: 10.1021/acschembio.9b00741 Chunyang Guo 1 , Lindsey K Steinberg 2 , Ming Cheng 1 , Jong Hee Song 1 , Jeffrey P Henderson 2 , Michael L Gross 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-01-13 , DOI: 10.1021/acschembio.9b00741 Chunyang Guo 1 , Lindsey K Steinberg 2 , Ming Cheng 1 , Jong Hee Song 1 , Jeffrey P Henderson 2 , Michael L Gross 1
Affiliation
|
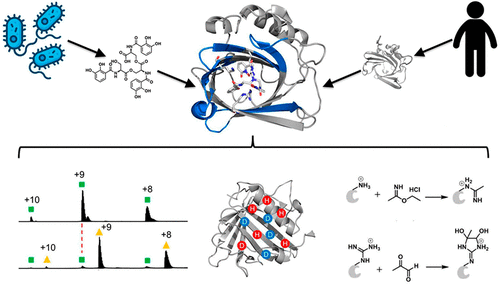
Both host and pathogen competitively manipulate coordination environments during bacterial infections. Human cells release the innate immune protein siderocalin (Scn, also known as lipocalin-2/Lcn2, neutrophil gelatinase-associated lipocalin/NGAL) that can inhibit bacterial growth by sequestering iron in a ferric complex with enterobactin (Ent), the ubiquitous Escherichia coli siderophore. Pathogenic E. coli use the virulence-associated esterase IroE to linearize the Ent cyclic trilactone to linear enterobactin (lin-Ent). We characterized lin-Ent interactions with Scn by using native mass spectrometry (MS) with hydrogen-deuterium exchange (HDX) and Lys/Arg specific covalent footprinting. These approaches support 1:1 binding of both Fe(III)-lin-Ent to Scn and iron-free lin-Ent to Scn. Both ferric and nonferric lin-Ent localize to all three pockets of the Scn calyx, consistent with Scn capture of lin-Ent both before and after Fe(III) chelation. These findings raise the possibility that Scn neutralizes both siderophores and siderophore-bound iron during infections. This integrated, MS-based approach circumvents the limitations that frustrate traditional structural approaches to examining Scn interactions with enterobactin-based ligands.
中文翻译:

质谱法揭示了位点特异性铁铁蛋白与铁和无铁肠杆菌素的结合。
在细菌感染期间,宿主和病原体都竞争性地操纵协调环境。人体细胞释放先天免疫蛋白铁运载蛋白(Scn,也称为脂质运载蛋白-2/Lcn2、中性粒细胞明胶酶相关脂质运载蛋白/NGAL),该蛋白可以通过与普遍存在的大肠杆菌(Escherichia coli)肠杆菌素(Ent)形成三价铁复合物来螯合铁,从而抑制细菌生长铁载体。致病性大肠杆菌使用毒力相关酯酶 IroE 将 Ent 环状三内酯线性化为线性肠杆菌素 (lin-Ent)。我们通过使用具有氢-氘交换 (HDX) 和 Lys/Arg 特异性共价足迹的天然质谱 (MS) 来表征 lin-Ent 与 Scn 的相互作用。这些方法支持 Fe(III)-lin-Ent 与 Scn 以及无铁 lin-Ent 与 Scn 的 1:1 结合。铁和非铁 lin-Ent 均定位于 Scn 花萼的所有三个口袋,这与 Fe(III) 螯合之前和之后 lin-Ent 的 Scn 捕获一致。这些发现提出了 Scn 在感染过程中中和铁载体和铁载体结合铁的可能性。这种基于 MS 的集成方法克服了传统结构方法检查 Scn 与肠杆菌素配体相互作用的局限性。
更新日期:2019-12-23
中文翻译:

质谱法揭示了位点特异性铁铁蛋白与铁和无铁肠杆菌素的结合。
在细菌感染期间,宿主和病原体都竞争性地操纵协调环境。人体细胞释放先天免疫蛋白铁运载蛋白(Scn,也称为脂质运载蛋白-2/Lcn2、中性粒细胞明胶酶相关脂质运载蛋白/NGAL),该蛋白可以通过与普遍存在的大肠杆菌(Escherichia coli)肠杆菌素(Ent)形成三价铁复合物来螯合铁,从而抑制细菌生长铁载体。致病性大肠杆菌使用毒力相关酯酶 IroE 将 Ent 环状三内酯线性化为线性肠杆菌素 (lin-Ent)。我们通过使用具有氢-氘交换 (HDX) 和 Lys/Arg 特异性共价足迹的天然质谱 (MS) 来表征 lin-Ent 与 Scn 的相互作用。这些方法支持 Fe(III)-lin-Ent 与 Scn 以及无铁 lin-Ent 与 Scn 的 1:1 结合。铁和非铁 lin-Ent 均定位于 Scn 花萼的所有三个口袋,这与 Fe(III) 螯合之前和之后 lin-Ent 的 Scn 捕获一致。这些发现提出了 Scn 在感染过程中中和铁载体和铁载体结合铁的可能性。这种基于 MS 的集成方法克服了传统结构方法检查 Scn 与肠杆菌素配体相互作用的局限性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号