当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Competing Interactions: Evolution of Inter and Intramolecular Hydrogen Bonds in Salicylic Acid at High Pressures.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2019-12-23 , DOI: 10.1021/acs.jpcb.9b10432 Ajay K Mishra 1 , Chitra Murli 1, 2 , K K Pandey 1 , T Sakuntala 2, 3 , Himanshu Kumar Poswal 1 , Ashok K Verma 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2019-12-23 , DOI: 10.1021/acs.jpcb.9b10432 Ajay K Mishra 1 , Chitra Murli 1, 2 , K K Pandey 1 , T Sakuntala 2, 3 , Himanshu Kumar Poswal 1 , Ashok K Verma 1
Affiliation
|
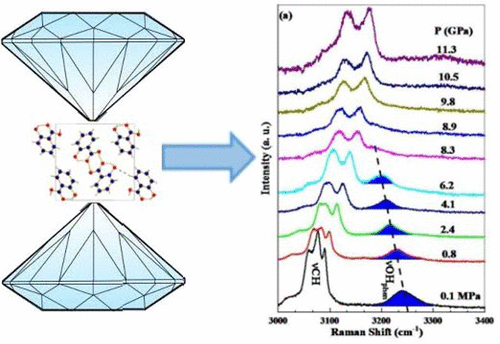
Benzoic acid derivatives are important molecular systems in the pharmaceutical industry. Salicylic acid is distinct among the derivatives of benzoic acid due to the presence of an intramolecular hydrogen bond. With a view to study the evolution of inter and intramolecular hydrogen bonding at shorter length scales, in situ high pressure Raman spectroscopic measurements, angle dispersive X-ray diffraction experiments, and density functional theory (DFT) based first principle calculations have been carried out on crystalline salicylic acid. Subtle structural modifications are noted across ∼1 GPa leading to structural phase transition to a new crystalline phase above 7 GPa which is reversible. Substantial softening of the OH stretching Raman mode associated with intramolecular hydrogen bond is observed prior to the transition pressure. Possible molecular configurations associated with tautomerization are discussed with the aid of DFT calculations.
中文翻译:

竞争性相互作用:高压下水杨酸中分子间和分子内氢键的演变。
苯甲酸衍生物是制药工业中重要的分子系统。由于存在分子内氢键,水杨酸在苯甲酸的衍生物之间是不同的。为了研究在较短的长度尺度上分子间和分子内氢键的演变,已经进行了基于原位高压拉曼光谱测量,角度色散X射线衍射实验和基于密度泛函理论(DFT)的第一原理计算。结晶水杨酸。在大约1 GPa的范围内发现了微妙的结构修饰,导致结构相转变为高于7 GPa的可逆的新结晶相。在转变压力之前,观察到与分子内氢键相关的OH拉伸拉曼模式的显着软化。
更新日期:2020-01-07
中文翻译:

竞争性相互作用:高压下水杨酸中分子间和分子内氢键的演变。
苯甲酸衍生物是制药工业中重要的分子系统。由于存在分子内氢键,水杨酸在苯甲酸的衍生物之间是不同的。为了研究在较短的长度尺度上分子间和分子内氢键的演变,已经进行了基于原位高压拉曼光谱测量,角度色散X射线衍射实验和基于密度泛函理论(DFT)的第一原理计算。结晶水杨酸。在大约1 GPa的范围内发现了微妙的结构修饰,导致结构相转变为高于7 GPa的可逆的新结晶相。在转变压力之前,观察到与分子内氢键相关的OH拉伸拉曼模式的显着软化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号