当前位置:
X-MOL 学术
›
ChemPhotoChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Photodynamics of Stilbenyl‐Azopyrroles: Two‐Photon Controllable Photoswitching Systems
ChemPhotoChem ( IF 3.0 ) Pub Date : 2019-10-08 , DOI: 10.1002/cptc.201900185 Leonardo Muñoz‐Rugeles 1 , David Gallardo‐Rosas 2 , Jesús Durán‐Hernández 1 , Rafael López‐Arteaga 1 , R. Alfredo Toscano 1 , Nuria Esturau‐Escofet 1 , José G. López‐Cortés 1 , Jorge Peón 1 , M. Carmen Ortega‐Alfaro 2
ChemPhotoChem ( IF 3.0 ) Pub Date : 2019-10-08 , DOI: 10.1002/cptc.201900185 Leonardo Muñoz‐Rugeles 1 , David Gallardo‐Rosas 2 , Jesús Durán‐Hernández 1 , Rafael López‐Arteaga 1 , R. Alfredo Toscano 1 , Nuria Esturau‐Escofet 1 , José G. López‐Cortés 1 , Jorge Peón 1 , M. Carmen Ortega‐Alfaro 2
Affiliation
|
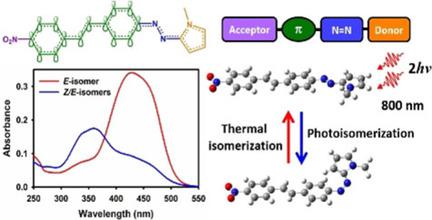
Three new stilbenyl‐azopyrroles 3 a–c were synthesized through a Mizoroki‐Heck C−C‐type coupling reaction between the 2‐(4′‐iodophenyl‐azo)‐N‐methyl pyrrole (1), and three different para‐substituted styrene derivatives. Inclusion of additional NO2 and NMe2 groups at the para‐position of the stilbenyl section resulted in significant changes of their linear and non‐linear optical properties. The photoisomerization behavior was studied through ultrafast laser techniques for single‐ and two‐photon excitation. The time‐resolved studies indicate that, despite the drastic changes in conjugation, the typical path for photoisomerization is maintained in these systems for both single and biphotonic excitation. In particular, the NO2 substituted molecule showed improved two‐photon absorption capacities, together with a significant trans−cis isomerization channel, implying that the photoswitching process can be controlled with high spatial precision through non‐linear optical excitation.
中文翻译:

苯乙烯-偶氮吡咯的合成和光动力学:两光子可控的光开关系统
通过2-(4'-碘苯基-偶氮)-N-甲基吡咯(1)和三种不同的对位取代基之间的Mizoroki-Heck C-C型偶联反应合成了三个新的二苯乙烯基-偶氮吡咯3a - c。苯乙烯衍生物。在第-段中包括额外的NO 2和NMe 2基团二苯乙烯基截面的位置导致其线性和非线性光学性质发生显着变化。通过超快激光技术对单光子和双光子激发进行了研究,研究了光致异构化行为。时间分辨研究表明,尽管共轭发生了巨大变化,但对于单光子激发和双光子激发,这些系统中均保持了典型的光异构化路径。特别是,NO 2取代的分子显示出改善的双光子吸收能力,以及显着的反式-顺式异构化通道,这意味着可以通过非线性光学激发以高空间精度控制光开关过程。
更新日期:2019-10-08
中文翻译:

苯乙烯-偶氮吡咯的合成和光动力学:两光子可控的光开关系统
通过2-(4'-碘苯基-偶氮)-N-甲基吡咯(1)和三种不同的对位取代基之间的Mizoroki-Heck C-C型偶联反应合成了三个新的二苯乙烯基-偶氮吡咯3a - c。苯乙烯衍生物。在第-段中包括额外的NO 2和NMe 2基团二苯乙烯基截面的位置导致其线性和非线性光学性质发生显着变化。通过超快激光技术对单光子和双光子激发进行了研究,研究了光致异构化行为。时间分辨研究表明,尽管共轭发生了巨大变化,但对于单光子激发和双光子激发,这些系统中均保持了典型的光异构化路径。特别是,NO 2取代的分子显示出改善的双光子吸收能力,以及显着的反式-顺式异构化通道,这意味着可以通过非线性光学激发以高空间精度控制光开关过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号