当前位置:
X-MOL 学术
›
ChemPhotoChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intra‐ and Intermolecular Fluorescence Quenching of Alkylthio‐Substituted Phthalimides by Photoinduced Electron Transfer: Distance, Position and Conformational Dependence
ChemPhotoChem ( IF 3.0 ) Pub Date : 2019-09-11 , DOI: 10.1002/cptc.201900175 Murat Atar 1 , Banu Öngel 1 , Henrik Riedasch 1 , Tim Lippold 1 , Jörg Neudörfl 1 , Diego Sampedro 2 , Axel G. Griesbeck 1
ChemPhotoChem ( IF 3.0 ) Pub Date : 2019-09-11 , DOI: 10.1002/cptc.201900175 Murat Atar 1 , Banu Öngel 1 , Henrik Riedasch 1 , Tim Lippold 1 , Jörg Neudörfl 1 , Diego Sampedro 2 , Axel G. Griesbeck 1
Affiliation
|
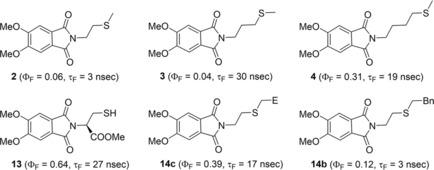
The photophysical properties of fluorescent phthalimides with thioether groups directly connected to the chromophore or separated by alkyl spacers, respectively, were studied. Intermolecular fluorescence quenching by electron transfer from dimethylsulfide to the 4,5‐dimethoxy phthalimide (DMPht) model compound 6 is dynamic and fast. The fluorescence properties of 6 and the remote C5‐spaced thioether derivative 5 are nearly identical. In compounds 1–4 with shorter spacer lengths, fluorescence quenching is strong for C2 and C3‐spaced 2 and 3 and less pronounced for C1‐ and C4‐spaced compounds 1 and 4, mapping the conformational landscape of these molecules. The o‐,m‐,p‐substituted N‐(thiomethyl)benzyl DMPht 7 are almost non‐fluorescent which correlates very well with the intermolecular thioanisole fluorescence quenching of 6. In contrast, the 3‐ and 4‐thiomethyl phthalimides 8 and 9 show divergent fluorescence that is also rationalized by DFT calculation results. The fluorescence properties can be switched by oxidation of the thioethers to sulfoxides with H2O2 or 1O2 (off→on for 1,4,7 and on→off for 9). Further functionalized molecules that were based on the model compounds are the sulfur‐containing amino acid methionine derivative 10, dipeptides 11 a,b, and S‐alkylated cysteine derivatives 12 to 14 a–d with strong side‐chain‐dependent fluorescence.
中文翻译:

光诱导电子转移对烷硫基取代邻苯二甲酰亚胺的分子内和分子间荧光猝灭:距离,位置和构象依赖性
研究了具有直接与生色团相连或被烷基间隔基隔开的硫醚基团的荧光邻苯二甲酰亚胺的光物理性质。通过电子从二甲基硫转移到4,5-二甲氧基邻苯二甲酰亚胺(DMPht)模型化合物6的分子间荧光猝灭是动态且快速的。6和远离C 5的硫醚衍生物5的荧光性质几乎相同。在化合物1 - 4具有较短间隔基长度,荧光猝灭对于C是强2和C 3 -spaced 2和3和不太明显对于C 1和C 4隔开的化合物1和4,绘制了这些分子的构象图。的ø - ,米- ,p取代的N-(硫代甲基)苄基DMPht 7几乎无荧光,其与分子间茴香硫醚荧光猝灭相关非常好6。相比之下,3-和4-硫代甲基邻苯二甲酰亚胺8和9显示出发散的荧光,这也可以通过DFT计算结果来合理化。可以通过用H 2 O 2或1 O将硫醚氧化为亚砜来切换荧光性质。2(OFF→ON为1,4,7和ON→OFF为9)。是基于模型化合物进一步官能化分子是氨基酸甲硫氨酸衍生物含硫10,二肽11 一个,b,和S-烷基化半胱氨酸衍生物12至14 一个- d与强侧链依赖性荧光。
更新日期:2019-09-11
中文翻译:

光诱导电子转移对烷硫基取代邻苯二甲酰亚胺的分子内和分子间荧光猝灭:距离,位置和构象依赖性
研究了具有直接与生色团相连或被烷基间隔基隔开的硫醚基团的荧光邻苯二甲酰亚胺的光物理性质。通过电子从二甲基硫转移到4,5-二甲氧基邻苯二甲酰亚胺(DMPht)模型化合物6的分子间荧光猝灭是动态且快速的。6和远离C 5的硫醚衍生物5的荧光性质几乎相同。在化合物1 - 4具有较短间隔基长度,荧光猝灭对于C是强2和C 3 -spaced 2和3和不太明显对于C 1和C 4隔开的化合物1和4,绘制了这些分子的构象图。的ø - ,米- ,p取代的N-(硫代甲基)苄基DMPht 7几乎无荧光,其与分子间茴香硫醚荧光猝灭相关非常好6。相比之下,3-和4-硫代甲基邻苯二甲酰亚胺8和9显示出发散的荧光,这也可以通过DFT计算结果来合理化。可以通过用H 2 O 2或1 O将硫醚氧化为亚砜来切换荧光性质。2(OFF→ON为1,4,7和ON→OFF为9)。是基于模型化合物进一步官能化分子是氨基酸甲硫氨酸衍生物含硫10,二肽11 一个,b,和S-烷基化半胱氨酸衍生物12至14 一个- d与强侧链依赖性荧光。











































 京公网安备 11010802027423号
京公网安备 11010802027423号