当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Interplay between Charge-Shift Bonding and Halogen Bonding.
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-01-09 , DOI: 10.1002/cphc.201901023 Serigne Sarr 1 , Jérôme Graton 1 , Gilles Montavon 2 , Julien Pilmé 3 , Nicolas Galland 1
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-01-09 , DOI: 10.1002/cphc.201901023 Serigne Sarr 1 , Jérôme Graton 1 , Gilles Montavon 2 , Julien Pilmé 3 , Nicolas Galland 1
Affiliation
|
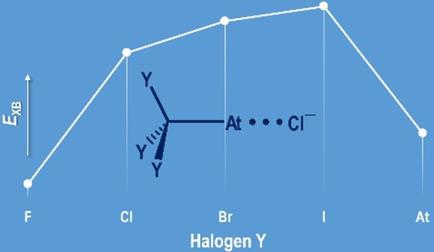
The nature of halogen‐bond interactions has been analysed from the perspective of the astatine element, which is potentially the strongest halogen‐bond donor. Relativistic quantum calculations on complexes formed between halide anions and a series of Y3C−X (Y=F to X, X=I, At) halogen‐bond donors disclosed unexpected trends, e. g., At3C−At revealing a weaker donating ability than I3C−I despite a stronger polarizability. All the observed peculiarities have their origin in a specific component of C−Y bonds: the charge‐shift bonding. Descriptors of the Quantum Chemical Topology show that the halogen‐bond strength can be quantitatively anticipated from the magnitude of charge‐shift bonding operating in Y3C−X. The charge‐shift mechanism weakens the ability of the halogen atom X to engage in halogen bonds. This outcome provides rationales for outlier halogen‐bond complexes, which are at variance with the consensus that the halogen‐bond strength scales with the polarizability of the halogen atom.
中文翻译:

关于电荷转移键和卤素键之间的相互作用。
从from元素的角度分析了卤素键相互作用的性质,a元素可能是最强的卤素键供体。对卤化物阴离子与一系列Y 3 C-X(Y = F to X,X = I,At)卤素键供体之间形成的络合物的相对论量子计算揭示了出乎意料的趋势,例如。g。,尽管极化能力较强,但At 3 C-At的捐赠能力却比I 3 C-I弱。所有观察到的特性都起源于CY键的特定组成部分:电荷移位键。量子化学拓扑学的描述表明,可以从Y 3中操作的电荷转移键的数量来定量预测卤素键的强度。C-X。电荷转移机制削弱了卤素原子X参与卤素键的能力。这一结果为离群的卤素键配合物提供了理论依据,这与卤素键强度随卤素原子的极化度成比例的共识不一致。
更新日期:2020-01-09
中文翻译:

关于电荷转移键和卤素键之间的相互作用。
从from元素的角度分析了卤素键相互作用的性质,a元素可能是最强的卤素键供体。对卤化物阴离子与一系列Y 3 C-X(Y = F to X,X = I,At)卤素键供体之间形成的络合物的相对论量子计算揭示了出乎意料的趋势,例如。g。,尽管极化能力较强,但At 3 C-At的捐赠能力却比I 3 C-I弱。所有观察到的特性都起源于CY键的特定组成部分:电荷移位键。量子化学拓扑学的描述表明,可以从Y 3中操作的电荷转移键的数量来定量预测卤素键的强度。C-X。电荷转移机制削弱了卤素原子X参与卤素键的能力。这一结果为离群的卤素键配合物提供了理论依据,这与卤素键强度随卤素原子的极化度成比例的共识不一致。









































 京公网安备 11010802027423号
京公网安备 11010802027423号