当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New pyrazole derivatives possessing amino/methanesulphonyl pharmacophore with good gastric safety profile: Design, synthesis, cyclooxygenase inhibition, anti-inflammatory activity and histopathological studies.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.bioorg.2019.103540 Khaled R A Abdellatif 1 , Eman K A Abdelall 2 , Phoebe F Lamie 2 , Madlen B Labib 2 , El-Shaymaa El-Nahaas 3 , Marwa M Abdelhakeem 2
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.bioorg.2019.103540 Khaled R A Abdellatif 1 , Eman K A Abdelall 2 , Phoebe F Lamie 2 , Madlen B Labib 2 , El-Shaymaa El-Nahaas 3 , Marwa M Abdelhakeem 2
Affiliation
|
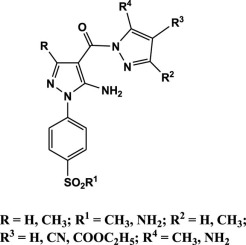
New series of pyrazole derivatives Va-c, VIa-c, VIIa-f, and VIII possessing amino/methanesulphonyl moiety as COX-2 pharmacophore were designed and synthesized. All compounds were evaluated for both in vitro COX inhibition and in vivo anti-inflammatory activities and all of them were more potent against COX-2 than COX-1 isozyme and showed good in vivo anti-inflammatory activity. Compounds Va, VIa, VIc and VIIa-c showed good COX-2 SI (246.8-353.8) in comparison with the COX-2 selective drug; celecoxib (326.7). Also, they showed good anti-inflammatory activity with edema inhibition (51-86 and 83-96%) relative to celecoxib (60.6 and 82.8%) after 3 and 5 h respectively. Additionally, these potent derivatives Va, VIa, VIc and VIIa-c were significantly less ulcerogenic (ulcer indexes = 0.7-2.0) than indomethacin (ulcer index = 21.3) and were of acceptable ulcerogenicity when compared with the non-ulcerogenic reference drug celecoxib (ulcer index = 1.3). The obtained ulcerogenic liability data revealed the gastric safety of these derivatives which was confirmed by the histopathological studies. Docking study was performed for all synthesized derivatives to explain their interaction with COX-2 receptor active site.
中文翻译:

具有氨基/甲磺酰基药效团并具有良好胃安全性的新型吡唑衍生物:设计,合成,环氧合酶抑制,抗炎活性和组织病理学研究。
设计并合成了具有氨基/甲磺酰基部分作为COX-2药效团的新系列吡唑衍生物Va-c,VIa-c,VIIa-f和VIII。评估了所有化合物的体外COX抑制作用和体内抗炎活性,并且它们均比COX-1同工酶对COX-2更有效,并显示出良好的体内抗炎活性。与COX-2选择性药物相比,化合物Va,VIa,VIc和VIIa-c显示出良好的COX-2 SI(246.8-353.8)。塞来昔布(326.7)。而且,相对于塞来昔布(60.6和82.8%),它们分别在3和5小时后显示出良好的抗炎活性,具有水肿抑制作用(51-86和83-96%)。此外,这些有效的衍生物Va,VIa,VIc和VIIa-c的致溃疡性(溃疡指数= 0.7-2.0)显着低于消炎痛(溃疡指数= 21)。3)与非致癌参考药物塞来昔布(溃疡指数= 1.3)相比具有可接受的致溃疡性。所获得的致溃疡性责任数据揭示了这些衍生物的胃安全性,这已被组织病理学研究证实。对所有合成衍生物进行了对接研究,以解释它们与COX-2受体活性位点的相互作用。
更新日期:2019-12-23
中文翻译:

具有氨基/甲磺酰基药效团并具有良好胃安全性的新型吡唑衍生物:设计,合成,环氧合酶抑制,抗炎活性和组织病理学研究。
设计并合成了具有氨基/甲磺酰基部分作为COX-2药效团的新系列吡唑衍生物Va-c,VIa-c,VIIa-f和VIII。评估了所有化合物的体外COX抑制作用和体内抗炎活性,并且它们均比COX-1同工酶对COX-2更有效,并显示出良好的体内抗炎活性。与COX-2选择性药物相比,化合物Va,VIa,VIc和VIIa-c显示出良好的COX-2 SI(246.8-353.8)。塞来昔布(326.7)。而且,相对于塞来昔布(60.6和82.8%),它们分别在3和5小时后显示出良好的抗炎活性,具有水肿抑制作用(51-86和83-96%)。此外,这些有效的衍生物Va,VIa,VIc和VIIa-c的致溃疡性(溃疡指数= 0.7-2.0)显着低于消炎痛(溃疡指数= 21)。3)与非致癌参考药物塞来昔布(溃疡指数= 1.3)相比具有可接受的致溃疡性。所获得的致溃疡性责任数据揭示了这些衍生物的胃安全性,这已被组织病理学研究证实。对所有合成衍生物进行了对接研究,以解释它们与COX-2受体活性位点的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号