当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and selective inhibitory effects of some 2-oxindole benzenesulfonamide conjugates on human carbonic anhydrase isoforms CA I, CA II, CA IX and CAXII.
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.bioorg.2019.103514 Riham F George 1 , Mona F Said 1 , Silvia Bua 2 , Claudiu T Supuran 2
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.bioorg.2019.103514 Riham F George 1 , Mona F Said 1 , Silvia Bua 2 , Claudiu T Supuran 2
Affiliation
|
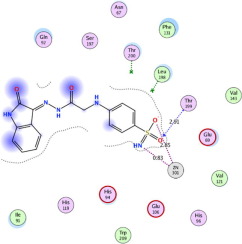
Three series of 2-oxindole benzenesulfonamide conjugates with different linkers were prepared by the condensation reaction of isatin derivatives 1a-e with different benzenesulfonamides. They were screened for their ability to inhibit human (h) carbonic anhydrase (CA, EC 4.2.1.1) isoforms hCA I, hCA II, hCA IX and hCA XII. Many compounds revealed promising activity and selectivity toward CAI, CAII and CAIX compared to acetazolamide (AAZ) especially compounds 2b (KI = 97.6, 8.0 nM against hCA I, hCA II, respectively) and 3a (KI = 90.2, 6.5 and 21.4 nM against hCA I, hCA II and hCA IX, respectively) relative to AAZ (KI = 250, 12 and 25 nM). Additionally, compound 4a revealed the highest activity against hCA II and hCA IX with KI of 3.0 and 13.9 nM, respectively. Docking of 2b, 3a and 4a into the active site of CA I, II, IX and XII revealed binding mode comparable to AAZ confirming the inhibition results.
中文翻译:

一些2-氧吲哚苯磺酰胺结合物的合成及其对人碳酸酐酶同工型CA I,CA II,CA IX和CAXII的选择性抑制作用。
通过使靛红衍生物1a-e与不同的苯磺酰胺缩合反应,制备了具有不同接头的三系列2-氧吲哚苯磺酰胺共轭物。对它们抑制人(h)碳酸酐酶(CA,EC 4.2.1.1)同工型hCA I,hCA II,hCA IX和hCA XII的能力进行了筛选。与乙酰唑胺(AAZ)相比,许多化合物显示出对CAI,CAII和CAIX有希望的活性和选择性,尤其是化合物2b(针对hCA I,hCA II分别为KI = 97.6、8.0 nM)和3a(针对hCA I,hCA II分别为KI = 90.2、6.5和21.4 nM hCA I,hCA II和hCA IX相对于AAZ(KI = 250、12和25 nM)。另外,化合物4a显示出针对hCA II和hCA IX的最高活性,KI分别为3.0和13.9 nM。将2b,3a和4a对接到CA I,II的活动站点,
更新日期:2019-12-23
中文翻译:

一些2-氧吲哚苯磺酰胺结合物的合成及其对人碳酸酐酶同工型CA I,CA II,CA IX和CAXII的选择性抑制作用。
通过使靛红衍生物1a-e与不同的苯磺酰胺缩合反应,制备了具有不同接头的三系列2-氧吲哚苯磺酰胺共轭物。对它们抑制人(h)碳酸酐酶(CA,EC 4.2.1.1)同工型hCA I,hCA II,hCA IX和hCA XII的能力进行了筛选。与乙酰唑胺(AAZ)相比,许多化合物显示出对CAI,CAII和CAIX有希望的活性和选择性,尤其是化合物2b(针对hCA I,hCA II分别为KI = 97.6、8.0 nM)和3a(针对hCA I,hCA II分别为KI = 90.2、6.5和21.4 nM hCA I,hCA II和hCA IX相对于AAZ(KI = 250、12和25 nM)。另外,化合物4a显示出针对hCA II和hCA IX的最高活性,KI分别为3.0和13.9 nM。将2b,3a和4a对接到CA I,II的活动站点,



























 京公网安备 11010802027423号
京公网安备 11010802027423号