Nature Catalysis ( IF 42.8 ) Pub Date : 2019-12-23 , DOI: 10.1038/s41929-019-0398-0 Song Song , Xinyao Li , Jialiang Wei , Weijin Wang , Yiqun Zhang , Lingsheng Ai , Yuchao Zhu , Xiaomeng Shi , Xiaohui Zhang , Ning Jiao
|
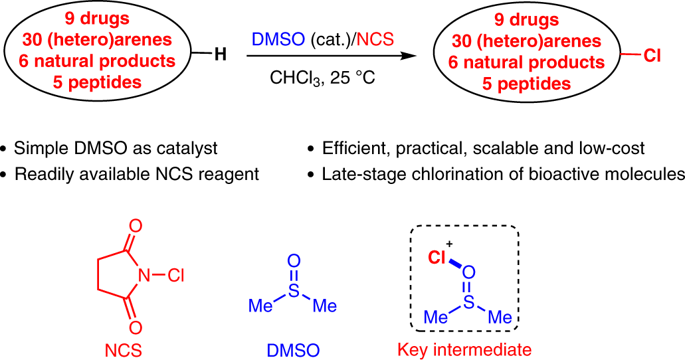
The chlorination of a bioactive compound can change its physiological properties and improve its pharmacokinetic and pharmacological profiles. It therefore has been an important strategy for drug discovery and development. However, the direct aromatic chlorination of complex bioactive molecules is too difficult to be practical. In fact, many functional groups such as hydroxyls, amines, amides or carboxylic acids may strongly restrain the reactivity of Cl+ by forming a halogen bond. Here we report a highly efficient aromatic chlorination of arenes that is catalysed by dimethyl sulfoxide with N-chlorosuccinimide as the chloro source. The mild conditions, easy-availability and stability of the catalyst and reagents, as well as good functional-group tolerance, showed the approach to be a versatile protocol for the late-stage aromatic chlorination of complex natural products, drugs and peptides. The multi-gram experiment and low-cost of N-chlorosuccinimide and dimethyl sulfoxide shows great potential for drug discovery and development in industrial applications.
中文翻译:

DMSO催化的(杂)芳烃的后期氯化
生物活性化合物的氯化可改变其生理特性,并改善其药代动力学和药理特性。因此,它已成为药物发现和开发的重要策略。然而,复杂的生物活性分子的直接芳族氯化反应太难于实际。实际上,许多官能团(例如羟基,胺,酰胺或羧酸)可以通过形成卤素键来强烈限制Cl +的反应性。在这里,我们报告了由二甲基亚砜和N催化的芳烃的高效芳烃氯化反应-氯代琥珀酰亚胺为氯源。温和的条件,催化剂和试剂的易用性和稳定性以及良好的官能团耐受性表明,该方法是复杂复杂天然产物,药物和多肽的后期芳香氯化的通用方法。N-氯代琥珀酰亚胺和二甲基亚砜的多克实验和低成本显示出在工业应用中发现和开发药物的巨大潜力。









































 京公网安备 11010802027423号
京公网安备 11010802027423号