当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Membrane Anchoring of Hck Kinase via the Intrinsically Disordered SH4-U and Length Scale Associated with Subcellular Localization.
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.jmb.2019.11.024 Matthew P Pond 1 , Rebecca Eells 2 , Bradley W Treece 2 , Frank Heinrich 3 , Mathias Lösche 4 , Benoît Roux 1
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2019-12-23 , DOI: 10.1016/j.jmb.2019.11.024 Matthew P Pond 1 , Rebecca Eells 2 , Bradley W Treece 2 , Frank Heinrich 3 , Mathias Lösche 4 , Benoît Roux 1
Affiliation
|
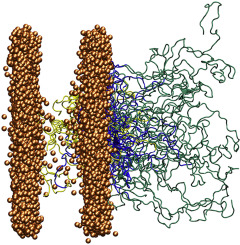
Src family kinases (SFKs) are a group of nonreceptor tyrosine kinases that are characterized by their involvement in critical signal transduction pathways. SFKs are often found attached to membranes, but little is known about the conformation of the protein in this environment. Here, solution nuclear magnetic resonance (NMR), neutron reflectometry (NR), and molecular dynamics (MD) simulations were employed to study the membrane interactions of the intrinsically disordered SH4 and Unique domains of the Src family kinase Hck. Through development of a procedure to combine the information from the different techniques, we were able produce a first-of-its-kind atomically detailed structural ensemble of a membrane-bound intrinsically disordered protein. Evaluation of the model demonstrated its consistency with previous work and provided insight into how SFK Unique domains act to differentiate the family members from one another. Fortuitously, the position of the ensemble on the membrane allowed the model to be combined with configurations of the multidomain Hck kinase previously determined from small-angle solution X-ray scattering to produce full-length models of membrane-anchored Hck. The resulting models allowed us to estimate that the kinase active site is positioned about 65 ± 35 Å away from the membrane surface, offering the first estimations of the length scale associated with the concept of SFK subcellular localization.
中文翻译:

Hck激酶通过固有紊乱的SH4-U和与亚细胞定位相关的长度尺度的膜锚定。
Src家族激酶(SFK)是一组非受体酪氨酸激酶,其特征在于它们参与关键信号转导途径。SFK通常被发现附着在膜上,但是对于这种环境下蛋白质的构象知之甚少。在这里,溶液核磁共振(NMR),中子反射法(NR)和分子动力学(MD)模拟被用来研究内在无序的SH4和Src家族激酶Hck的独特域的膜相互作用。通过开发一种程序来结合来自不同技术的信息,我们能够生产出膜结合的内在无序蛋白的首创的原子详细的结构整体。对模型的评估证明了其与先前工作的一致性,并提供了对SFK唯一域如何使家庭成员彼此区分的见解。幸运的是,膜上整体的位置使模型可以与先前由小角度溶液X射线散射确定的多域Hck激酶构型结合起来,从而产生膜锚定的Hck全长模型。所得模型使我们能够估计激酶活性位点位于距膜表面约65±35Å的位置,从而提供了与SFK亚细胞定位概念相关的长度尺度的首次估计。膜上集合体的位置使模型可以与先前通过小角度溶液X射线散射确定的多域Hck激酶构型结合起来,以产生全长的膜锚定Hck模型。所得模型使我们能够估计激酶活性位点位于距膜表面约65±35Å的位置,从而提供了与SFK亚细胞定位概念相关的长度尺度的首次估计。膜上集合体的位置使模型可以与先前通过小角度溶液X射线散射确定的多域Hck激酶构型结合起来,以产生全长的膜锚定Hck模型。所得模型使我们能够估计激酶活性位点位于距膜表面约65±35Å的位置,从而提供了与SFK亚细胞定位概念相关的长度尺度的首次估计。
更新日期:2019-12-23
中文翻译:

Hck激酶通过固有紊乱的SH4-U和与亚细胞定位相关的长度尺度的膜锚定。
Src家族激酶(SFK)是一组非受体酪氨酸激酶,其特征在于它们参与关键信号转导途径。SFK通常被发现附着在膜上,但是对于这种环境下蛋白质的构象知之甚少。在这里,溶液核磁共振(NMR),中子反射法(NR)和分子动力学(MD)模拟被用来研究内在无序的SH4和Src家族激酶Hck的独特域的膜相互作用。通过开发一种程序来结合来自不同技术的信息,我们能够生产出膜结合的内在无序蛋白的首创的原子详细的结构整体。对模型的评估证明了其与先前工作的一致性,并提供了对SFK唯一域如何使家庭成员彼此区分的见解。幸运的是,膜上整体的位置使模型可以与先前由小角度溶液X射线散射确定的多域Hck激酶构型结合起来,从而产生膜锚定的Hck全长模型。所得模型使我们能够估计激酶活性位点位于距膜表面约65±35Å的位置,从而提供了与SFK亚细胞定位概念相关的长度尺度的首次估计。膜上集合体的位置使模型可以与先前通过小角度溶液X射线散射确定的多域Hck激酶构型结合起来,以产生全长的膜锚定Hck模型。所得模型使我们能够估计激酶活性位点位于距膜表面约65±35Å的位置,从而提供了与SFK亚细胞定位概念相关的长度尺度的首次估计。膜上集合体的位置使模型可以与先前通过小角度溶液X射线散射确定的多域Hck激酶构型结合起来,以产生全长的膜锚定Hck模型。所得模型使我们能够估计激酶活性位点位于距膜表面约65±35Å的位置,从而提供了与SFK亚细胞定位概念相关的长度尺度的首次估计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号