当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ Acetaldehyde Synthesis for Carboligation Reactions.
ChemBioChem ( IF 2.6 ) Pub Date : 2020-02-12 , DOI: 10.1002/cbic.201900666 Lieuwe Biewenga 1 , Andreas Kunzendorf 1 , Gerrit J Poelarends 1
ChemBioChem ( IF 2.6 ) Pub Date : 2020-02-12 , DOI: 10.1002/cbic.201900666 Lieuwe Biewenga 1 , Andreas Kunzendorf 1 , Gerrit J Poelarends 1
Affiliation
|
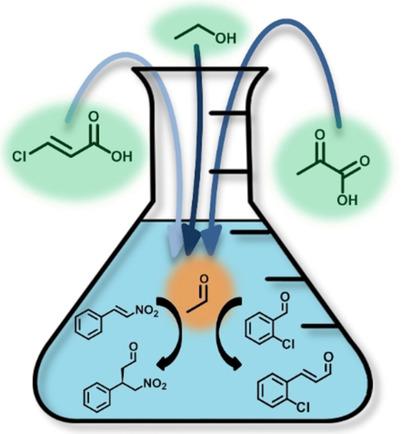
The enzyme 4-oxalocrotonate tautomerase (4-OT) can promiscuously catalyze various carboligation reactions using acetaldehyde as a nucleophile. However, the highly reactive nature of acetaldehyde requires intricate handling, which can impede its usage in practical synthesis. Therefore, we investigated three enzymatic routes to synthesize acetaldehyde in situ in one-pot cascade reactions with 4-OT. Two routes afforded practical acetaldehyde concentrations, using an environmental pollutant, trans-3-chloroacrylic acid, or a bio-renewable, ethanol, as starting substrate. These routes can be combined with 4-OT catalyzed Michael-type additions and aldol condensations in one pot. This modular systems biocatalysis methodology provides a stepping stone towards the development of larger artificial metabolic networks for the practical synthesis of important chemical synthons.
中文翻译:

用于碳化反应的原位乙醛合成。
4-草酰巴豆酸互变异构酶 (4-OT) 可以使用乙醛作为亲核试剂随意催化各种碳化反应。然而,乙醛的高反应性需要复杂的处理,这可能会阻碍其在实际合成中的使用。因此,我们研究了三种酶促路线,在与 4-OT 的一锅级联反应中原位合成乙醛。两种途径提供了实用的乙醛浓度,使用环境污染物反式3-氯丙烯酸或生物可再生乙醇作为起始底物。这些路线可以与 4-OT 催化的迈克尔型加成和羟醛缩合结合在一锅中。这种模块化系统生物催化方法为开发更大的人工代谢网络以实际合成重要的化学合成子提供了踏脚石。
更新日期:2019-12-23
中文翻译:

用于碳化反应的原位乙醛合成。
4-草酰巴豆酸互变异构酶 (4-OT) 可以使用乙醛作为亲核试剂随意催化各种碳化反应。然而,乙醛的高反应性需要复杂的处理,这可能会阻碍其在实际合成中的使用。因此,我们研究了三种酶促路线,在与 4-OT 的一锅级联反应中原位合成乙醛。两种途径提供了实用的乙醛浓度,使用环境污染物反式3-氯丙烯酸或生物可再生乙醇作为起始底物。这些路线可以与 4-OT 催化的迈克尔型加成和羟醛缩合结合在一锅中。这种模块化系统生物催化方法为开发更大的人工代谢网络以实际合成重要的化学合成子提供了踏脚石。











































 京公网安备 11010802027423号
京公网安备 11010802027423号