当前位置:
X-MOL 学术
›
Eur. Polym. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polysaccharide-fullerene supramolecular hybrids: synthesis, characterization and antioxidant activity
European Polymer Journal ( IF 5.8 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.eurpolymj.2019.109461 Tatjana J. Kop , Dragica M. Jakovljević , Ljiljana S. Živković , Andrijana Žekić , Vladimir P. Beškoski , Dragana R. Milić , Gordana D. Gojgić-Cvijović , Mira S. Bjelaković
European Polymer Journal ( IF 5.8 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.eurpolymj.2019.109461 Tatjana J. Kop , Dragica M. Jakovljević , Ljiljana S. Živković , Andrijana Žekić , Vladimir P. Beškoski , Dragana R. Milić , Gordana D. Gojgić-Cvijović , Mira S. Bjelaković
|
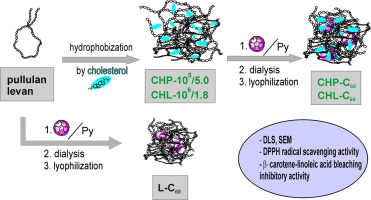
Abstract An efficient encapsulation of the fullerene into two hydrophobized and one native polysaccharide provided water soluble supramolecular hybrids. After covalent modification of polysaccharides by cholesterol, noncovalent hybrids were prepared by a three-step procedure, including mixing of individual aqueous solutions of hydrophobized, as well as native sugar with solution of the fullerene in pyridine, dialysis and lyophilization. Although the degree of the fullerene incorporation into hydrophobized substrates, cholesterol-levan and cholesterol-pullulan, was lower in comparison to the native polysaccharide levan, hydrophobization provided nanoparticles with improved properties. The particle size distribution, studied by dynamic light scattering and scanning electron microscopy revealed formation of moderately polydisperse aggregates, with the diameter contraction in comparison to the corresponding free polysaccharide, especially in the case of hydrophobized substrates. The morphological examination, done by scanning electron microscopy indicated the self-organization of the fullerene-native polysaccharide to round individual structures, while fullerene-hydrophobized polysaccharide hybrids tend to form networks. The antioxidant activity of the synthesized polysaccharide-C60 noncovalent hybrids versus starting polysaccharides was investigated by the DPPH radical scavenging and the β-carotene-linoleic acid bleaching methods. In all three complexes, the radical scavenging ability of the fullerene remained preserved, and a positive effect of levan hydrophobization was observed.
中文翻译:

多糖-富勒烯超分子杂化物:合成、表征和抗氧化活性
摘要 将富勒烯有效封装成两种疏水化多糖和一种天然多糖提供了水溶性超分子杂化物。通过胆固醇对多糖进行共价修饰后,通过三步程序制备非共价杂交体,包括将单独的疏水化水溶液以及天然糖与富勒烯的吡啶溶液混合、透析和冻干。尽管与天然多糖左聚糖相比,富勒烯掺入疏水化底物胆固醇-左聚糖和胆固醇-支链淀粉的程度较低,但疏水化为纳米颗粒提供了改进的性能。通过动态光散射和扫描电子显微镜研究的粒度分布表明形成了中等多分散的聚集体,与相应的游离多糖相比,直径收缩,特别是在疏水化底物的情况下。通过扫描电子显微镜进行的形态学检查表明,富勒烯天然多糖自组织形成圆形单个结构,而富勒烯疏水化多糖杂化物倾向于形成网络。通过DPPH自由基清除和β-胡萝卜素-亚油酸漂白方法研究了合成的多糖-C60非共价杂化物相对于起始多糖的抗氧化活性。在所有三种配合物中,富勒烯的自由基清除能力保持不变,并且观察到果聚糖疏水化的积极作用。特别是在疏水化基材的情况下。通过扫描电子显微镜进行的形态学检查表明,富勒烯天然多糖自组织形成圆形单个结构,而富勒烯疏水化多糖杂化物倾向于形成网络。通过DPPH自由基清除和β-胡萝卜素-亚油酸漂白方法研究了合成的多糖-C60非共价杂化物相对于起始多糖的抗氧化活性。在所有三种配合物中,富勒烯的自由基清除能力保持不变,并且观察到果聚糖疏水化的积极作用。特别是在疏水化基材的情况下。通过扫描电子显微镜进行的形态学检查表明,富勒烯天然多糖自组织形成圆形单个结构,而富勒烯疏水化多糖杂化物倾向于形成网络。通过DPPH自由基清除和β-胡萝卜素-亚油酸漂白方法研究了合成的多糖-C60非共价杂化物相对于起始多糖的抗氧化活性。在所有三种配合物中,富勒烯的自由基清除能力保持不变,并且观察到果聚糖疏水化的积极作用。通过扫描电子显微镜完成的研究表明,富勒烯天然多糖自组织形成单个结构,而富勒烯疏水化多糖杂化物则倾向于形成网络。通过DPPH自由基清除和β-胡萝卜素-亚油酸漂白方法研究了合成的多糖-C60非共价杂化物相对于起始多糖的抗氧化活性。在所有三种配合物中,富勒烯的自由基清除能力保持不变,并且观察到果聚糖疏水化的积极作用。通过扫描电子显微镜完成的研究表明,富勒烯天然多糖自组织形成单个结构,而富勒烯疏水化多糖杂化物则倾向于形成网络。通过DPPH自由基清除和β-胡萝卜素-亚油酸漂白方法研究了合成的多糖-C60非共价杂化物相对于起始多糖的抗氧化活性。在所有三种配合物中,富勒烯的自由基清除能力保持不变,并且观察到果聚糖疏水化的积极作用。通过DPPH自由基清除和β-胡萝卜素-亚油酸漂白方法研究了合成的多糖-C60非共价杂化物相对于起始多糖的抗氧化活性。在所有三种配合物中,富勒烯的自由基清除能力保持不变,并且观察到果聚糖疏水化的积极作用。通过DPPH自由基清除和β-胡萝卜素-亚油酸漂白方法研究了合成的多糖-C60非共价杂化物相对于起始多糖的抗氧化活性。在所有三种配合物中,富勒烯的自由基清除能力保持不变,并且观察到果聚糖疏水化的积极作用。
更新日期:2020-01-01
中文翻译:

多糖-富勒烯超分子杂化物:合成、表征和抗氧化活性
摘要 将富勒烯有效封装成两种疏水化多糖和一种天然多糖提供了水溶性超分子杂化物。通过胆固醇对多糖进行共价修饰后,通过三步程序制备非共价杂交体,包括将单独的疏水化水溶液以及天然糖与富勒烯的吡啶溶液混合、透析和冻干。尽管与天然多糖左聚糖相比,富勒烯掺入疏水化底物胆固醇-左聚糖和胆固醇-支链淀粉的程度较低,但疏水化为纳米颗粒提供了改进的性能。通过动态光散射和扫描电子显微镜研究的粒度分布表明形成了中等多分散的聚集体,与相应的游离多糖相比,直径收缩,特别是在疏水化底物的情况下。通过扫描电子显微镜进行的形态学检查表明,富勒烯天然多糖自组织形成圆形单个结构,而富勒烯疏水化多糖杂化物倾向于形成网络。通过DPPH自由基清除和β-胡萝卜素-亚油酸漂白方法研究了合成的多糖-C60非共价杂化物相对于起始多糖的抗氧化活性。在所有三种配合物中,富勒烯的自由基清除能力保持不变,并且观察到果聚糖疏水化的积极作用。特别是在疏水化基材的情况下。通过扫描电子显微镜进行的形态学检查表明,富勒烯天然多糖自组织形成圆形单个结构,而富勒烯疏水化多糖杂化物倾向于形成网络。通过DPPH自由基清除和β-胡萝卜素-亚油酸漂白方法研究了合成的多糖-C60非共价杂化物相对于起始多糖的抗氧化活性。在所有三种配合物中,富勒烯的自由基清除能力保持不变,并且观察到果聚糖疏水化的积极作用。特别是在疏水化基材的情况下。通过扫描电子显微镜进行的形态学检查表明,富勒烯天然多糖自组织形成圆形单个结构,而富勒烯疏水化多糖杂化物倾向于形成网络。通过DPPH自由基清除和β-胡萝卜素-亚油酸漂白方法研究了合成的多糖-C60非共价杂化物相对于起始多糖的抗氧化活性。在所有三种配合物中,富勒烯的自由基清除能力保持不变,并且观察到果聚糖疏水化的积极作用。通过扫描电子显微镜完成的研究表明,富勒烯天然多糖自组织形成单个结构,而富勒烯疏水化多糖杂化物则倾向于形成网络。通过DPPH自由基清除和β-胡萝卜素-亚油酸漂白方法研究了合成的多糖-C60非共价杂化物相对于起始多糖的抗氧化活性。在所有三种配合物中,富勒烯的自由基清除能力保持不变,并且观察到果聚糖疏水化的积极作用。通过扫描电子显微镜完成的研究表明,富勒烯天然多糖自组织形成单个结构,而富勒烯疏水化多糖杂化物则倾向于形成网络。通过DPPH自由基清除和β-胡萝卜素-亚油酸漂白方法研究了合成的多糖-C60非共价杂化物相对于起始多糖的抗氧化活性。在所有三种配合物中,富勒烯的自由基清除能力保持不变,并且观察到果聚糖疏水化的积极作用。通过DPPH自由基清除和β-胡萝卜素-亚油酸漂白方法研究了合成的多糖-C60非共价杂化物相对于起始多糖的抗氧化活性。在所有三种配合物中,富勒烯的自由基清除能力保持不变,并且观察到果聚糖疏水化的积极作用。通过DPPH自由基清除和β-胡萝卜素-亚油酸漂白方法研究了合成的多糖-C60非共价杂化物相对于起始多糖的抗氧化活性。在所有三种配合物中,富勒烯的自由基清除能力保持不变,并且观察到果聚糖疏水化的积极作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号