当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lateral size dependent colloidal stability of graphene oxide in water: impacts of protein properties and water chemistry
Environmental Science: Nano ( IF 7.3 ) Pub Date : 2019/12/23 , DOI: 10.1039/c9en01040h Binbin Sun 1, 2, 3, 4, 5 , Yinqing Zhang 1, 2, 3, 4, 5 , Qing Liu 1, 2, 3, 4, 5 , Chaorui Yan 6, 7, 8, 9 , Bowen Xiao 1, 2, 3, 4, 5 , Jing Yang 1, 2, 3, 4, 5 , Menglin Liu 1, 2, 3, 4, 5 , Lingyan Zhu 1, 2, 3, 4, 5
Environmental Science: Nano ( IF 7.3 ) Pub Date : 2019/12/23 , DOI: 10.1039/c9en01040h Binbin Sun 1, 2, 3, 4, 5 , Yinqing Zhang 1, 2, 3, 4, 5 , Qing Liu 1, 2, 3, 4, 5 , Chaorui Yan 6, 7, 8, 9 , Bowen Xiao 1, 2, 3, 4, 5 , Jing Yang 1, 2, 3, 4, 5 , Menglin Liu 1, 2, 3, 4, 5 , Lingyan Zhu 1, 2, 3, 4, 5
Affiliation
|
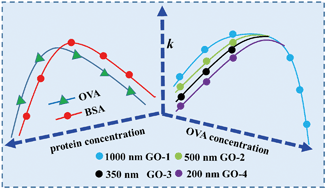
The effects of model proteins (OVA: ovalbumin and BSA: bovine serum albumin) on the colloidal stability of graphene oxide (GO) with different lateral sizes (around 1000, 500, 350 and 200 nm, respectively) were investigated. At low salt concentrations, the aggregation rate (k) of GO displayed a parabolic relationship: it increased until kfast (regime I), remained at kfast (regime II), and then decreased gradually to zero (regime III), with the increase of protein concentrations at environmental pH (4, 6 and 9). Compared to BSA, much higher concentrations of OVA were needed to achieve similar effects on GO stability induced by BSA, since OVA has a smaller molecular size and fewer positively charged groups. In regime I, the k of GO increased with the increase of lateral size at the same concentration of proteins, and a higher dosage of proteins was needed to drive k to kfast for smaller sized GO. However, there was no such lateral size dependent effect in regime III, which was attributed to the predominance of steric repulsion caused by the large amount of proteins. In regime I, GO displayed higher stability at higher pH, and thus more proteins were required to drive k to kfast. In regime III, the proteins were more effective in stabilizing GO under higher pH conditions due to the enhanced electrostatic and steric repulsion. This study highlights the crucial effects of the GO lateral size, protein properties and concentrations, as well as solution chemistry, on GO stability in aquatic environments.
中文翻译:

氧化石墨烯在水中的横向尺寸依赖性胶体稳定性:蛋白质性质和水化学的影响
研究了模型蛋白(OVA:卵清蛋白和BSA:牛血清白蛋白)对不同横向尺寸(分别为1000、500、350和200 nm左右)的氧化石墨烯(GO)的胶体稳定性的影响。在低盐浓度下,GO的聚集速率(k)显示出抛物线关系:它增加直到k快(方案I),保持为k快(方案II),然后逐渐降低至零(方案III),在环境pH值(4、6和9)下蛋白质浓度增加。与BSA相比,由于OVA具有较小的分子大小和较少的带正电基团,因此需要更高浓度的OVA才能达到由BSA诱导的GO稳定性的类似作用。在政权一中ķ GO与在蛋白的浓度相同的横向尺寸的增加而增加,并且是需要的驱动蛋白的较高剂量ķ到ķ快速为更小尺寸的GO。但是,在方案III中没有这种横向尺寸依赖性效应,这归因于大量蛋白质引起的空间排斥。在方案I中,GO在较高的pH值下显示出更高的稳定性,因此需要更多的蛋白质来快速驱动k到k。在方案III中,由于增强的静电和空间排斥力,蛋白质在较高pH条件下更稳定GO。这项研究强调了GO横向尺寸,蛋白质特性和浓度以及溶液化学对水生环境GO稳定性的关键影响。
更新日期:2020-02-20
中文翻译:

氧化石墨烯在水中的横向尺寸依赖性胶体稳定性:蛋白质性质和水化学的影响
研究了模型蛋白(OVA:卵清蛋白和BSA:牛血清白蛋白)对不同横向尺寸(分别为1000、500、350和200 nm左右)的氧化石墨烯(GO)的胶体稳定性的影响。在低盐浓度下,GO的聚集速率(k)显示出抛物线关系:它增加直到k快(方案I),保持为k快(方案II),然后逐渐降低至零(方案III),在环境pH值(4、6和9)下蛋白质浓度增加。与BSA相比,由于OVA具有较小的分子大小和较少的带正电基团,因此需要更高浓度的OVA才能达到由BSA诱导的GO稳定性的类似作用。在政权一中ķ GO与在蛋白的浓度相同的横向尺寸的增加而增加,并且是需要的驱动蛋白的较高剂量ķ到ķ快速为更小尺寸的GO。但是,在方案III中没有这种横向尺寸依赖性效应,这归因于大量蛋白质引起的空间排斥。在方案I中,GO在较高的pH值下显示出更高的稳定性,因此需要更多的蛋白质来快速驱动k到k。在方案III中,由于增强的静电和空间排斥力,蛋白质在较高pH条件下更稳定GO。这项研究强调了GO横向尺寸,蛋白质特性和浓度以及溶液化学对水生环境GO稳定性的关键影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号