当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cosolvent action of promethazine hydrochloride in (ethyl acetate + alcohols) and thermodynamic property analysis
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jct.2019.106030 Zehui Yang , Danfeng Shao , Guoquan Zhou
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jct.2019.106030 Zehui Yang , Danfeng Shao , Guoquan Zhou
|
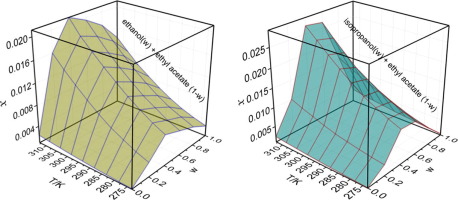
Abstract This study presents the solubility profiles of promethazine hydrochloride in pure and mixed solvents. The effects of temperature and composition of mixed solvents on solubility were investigated. Maximum solubility value was obtained in n-propanol in pure solvents, and in ethyl acetate, it is the lowest. While in two mixtures of ethyl acetate (1 − w) + ethanol (w, mass fraction) and ethyl acetate (1 − w) + isopropanol (w), the values increased monotonically with the increasing temperature, and increased with increasing co-solvent (alcohols) mass fraction (w) to a maximum value at w = 0.4 and then decreased. The interaction between promethazine hydrochloride molecule and solvent molecules was discussed. The function of temperature and solvent composition in pure and binary mixed solvents was evaluated by some thermodynamic models. The dissolution thermodynamic properties, including enthalpy, entropy and Gibbs energy of promethazine hydrochloride in the dissolution process were evaluated using the van't Hoff equation. The results indicate that in all selected solvents the dissolution behavior were endothermic and entropy-driven.
中文翻译:

盐酸异丙嗪在(乙酸乙酯+醇)中的共溶剂作用及热力学性质分析
摘要 本研究介绍了盐酸异丙嗪在纯溶剂和混合溶剂中的溶解度曲线。研究了温度和混合溶剂组成对溶解度的影响。在纯溶剂中的正丙醇中溶解度值最大,在乙酸乙酯中最低。而在乙酸乙酯 (1 − w) + 乙醇 (w, 质量分数) 和乙酸乙酯 (1 − w) + 异丙醇 (w) 的两种混合物中,这些值随着温度的升高而单调增加,并随着助溶剂的增加而增加(醇) 质量分数 (w) 在 w = 0.4 时达到最大值,然后下降。讨论了盐酸异丙嗪分子与溶剂分子之间的相互作用。通过一些热力学模型评估了纯溶剂和二元混合溶剂中温度和溶剂组成的作用。采用van't Hoff方程对盐酸异丙嗪在溶解过程中的焓、熵和吉布斯能等溶解热力学性质进行评价。结果表明,在所有选定的溶剂中,溶解行为都是吸热和熵驱动的。
更新日期:2020-04-01
中文翻译:

盐酸异丙嗪在(乙酸乙酯+醇)中的共溶剂作用及热力学性质分析
摘要 本研究介绍了盐酸异丙嗪在纯溶剂和混合溶剂中的溶解度曲线。研究了温度和混合溶剂组成对溶解度的影响。在纯溶剂中的正丙醇中溶解度值最大,在乙酸乙酯中最低。而在乙酸乙酯 (1 − w) + 乙醇 (w, 质量分数) 和乙酸乙酯 (1 − w) + 异丙醇 (w) 的两种混合物中,这些值随着温度的升高而单调增加,并随着助溶剂的增加而增加(醇) 质量分数 (w) 在 w = 0.4 时达到最大值,然后下降。讨论了盐酸异丙嗪分子与溶剂分子之间的相互作用。通过一些热力学模型评估了纯溶剂和二元混合溶剂中温度和溶剂组成的作用。采用van't Hoff方程对盐酸异丙嗪在溶解过程中的焓、熵和吉布斯能等溶解热力学性质进行评价。结果表明,在所有选定的溶剂中,溶解行为都是吸热和熵驱动的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号