Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Shank3 Binds to and Stabilizes the Active Form of Rap1 and HRas GTPases via Its NTD-ANK Tandem with Distinct Mechanisms.
Structure ( IF 4.4 ) Pub Date : 2019-12-17 , DOI: 10.1016/j.str.2019.11.018 Qixu Cai 1 , Tomohisa Hosokawa 2 , Menglong Zeng 1 , Yasunori Hayashi 2 , Mingjie Zhang 3
Structure ( IF 4.4 ) Pub Date : 2019-12-17 , DOI: 10.1016/j.str.2019.11.018 Qixu Cai 1 , Tomohisa Hosokawa 2 , Menglong Zeng 1 , Yasunori Hayashi 2 , Mingjie Zhang 3
Affiliation
|
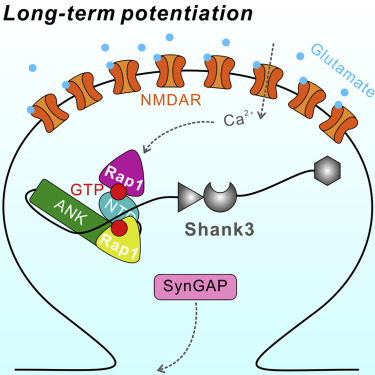
Shank1/2/3, major scaffold proteins in excitatory synapses, are frequently mutated in patients with psychiatric disorders. Although the Shank N-terminal domain and ankyrin repeats domain tandem (NTD-ANK) is known to bind to Ras and Rap1, the molecular mechanism underlying and functional significance of the bindings in synapses are unknown. Here, we demonstrate that Shank3 NTD-ANK specifically binds to the guanosine triphosphate (GTP)-bound form of HRas and Rap1. In addition to the canonical site mediated by the Ras-association domain and common to both GTPases, Shank3 contains an unconventional Rap1 binding site formed by NTD and ANK together. Binding of Shank3 to the GTP-loaded Rap1 slows down its GTP hydrolysis by SynGAP. We further show that the interactions between Shank3 and HRas/Rap1 at excitatory synapses are promoted by synaptic activation. Thus, Shank3 may be able to modulate signaling of the Ras family proteins via directly binding to and stabilizing the GTP-bound form of the enzymes.
中文翻译:

Shank3通过具有独特机制的NTD-ANK串联结合并稳定Rap1和HRas GTPases的活性形式。
Shank1 / 2/3是兴奋性突触中的主要支架蛋白,在患有精神病的患者中经常发生突变。尽管已知Shank N末端结构域和锚蛋白重复结构域串联(NTD-ANK)与Ras和Rap1结合,但是突触中结合的潜在分子机制和功能意义尚不清楚。在这里,我们证明Shank3 NTD-ANK特异性结合到HRas和Rap1的鸟苷三磷酸(GTP)结合形式。除了由Ras关联结构域介导且对两个GTPases通用的规范位点外,Shank3还包含由NTD和ANK共同形成的非常规Rap1结合位点。Shank3与装载GTP的Rap1的结合减慢了SynGAP水解GTP的速度。我们进一步表明,在兴奋性突触中Shank3和HRas / Rap1之间的相互作用是通过突触激活来促进的。
更新日期:2019-12-23
中文翻译:

Shank3通过具有独特机制的NTD-ANK串联结合并稳定Rap1和HRas GTPases的活性形式。
Shank1 / 2/3是兴奋性突触中的主要支架蛋白,在患有精神病的患者中经常发生突变。尽管已知Shank N末端结构域和锚蛋白重复结构域串联(NTD-ANK)与Ras和Rap1结合,但是突触中结合的潜在分子机制和功能意义尚不清楚。在这里,我们证明Shank3 NTD-ANK特异性结合到HRas和Rap1的鸟苷三磷酸(GTP)结合形式。除了由Ras关联结构域介导且对两个GTPases通用的规范位点外,Shank3还包含由NTD和ANK共同形成的非常规Rap1结合位点。Shank3与装载GTP的Rap1的结合减慢了SynGAP水解GTP的速度。我们进一步表明,在兴奋性突触中Shank3和HRas / Rap1之间的相互作用是通过突触激活来促进的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号